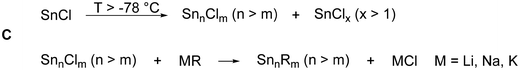Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Overview and perspectives on metalloid tin cluster chemistry
R.
Kimmich
and
A.
Schnepf
 *
*
Chemistry Department, University of Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany. E-mail: andreas.schnepf@uni-tuebingen.de
First published on 29th August 2024
Abstract
Although the first metalloid tin cluster was discovered by Wiberg in 1999, the number of isolated and characterized compounds is still low. However, numerous theoretical calculations indicate that a large variety of compounds are yet to be discovered, thereby suggesting that larger clusters might form a cluster-of-clusters arrangement rather than compact structures. This trend seems to be supported by the largest metalloid tin clusters exhibiting up to 20 tin atoms. In this review, recent results and future possibilities of this fascinating class of metalloid tin cluster compounds are discussed.
Introduction
The investigation of subvalent compounds of the heavier group 14 element tin is ongoing for a considerable period of time owing to the fascinating properties of these compounds. In the 1990s, Sita and co-workers discovered [1.1.1]propellanes with unusual bonding features, which exhibit tin(0) atoms capped by stannylene units.1–3 Additionally, they were able to synthesize interesting new cluster compounds using tin precursors as building blocks for more complex molecules via a wide range of reactions.4–6 In the following years, metal clusters gained more popularity, and the interest in the synthesis and characterization of metal-rich compounds increased. Through the input of Schnöckel and co-workers, metalloid clusters, especially those of group 13 elements aluminium and gallium, became a fruitful field of research to gain insights into the fundamental processes of the formation and dissolution of metals from/to molecular compounds.7 Thus, metal-rich metalloid clusters can be seen as ideal model compounds for this molecular area and are often stabilized by bulky ligands, partly exhibiting structural motives already known from the solid-state structure of the metal itself. Hence, these clusters are perfect, atomically precise model compounds for metal nanoparticles. In the field of metalloid tin clusters, the number of compounds is still quite low to date. Nevertheless, theoretical calculations and recent experimental results indicate that during the formation of larger clusters or nanoparticles with dozens to hundreds of tin atoms in the cluster core, an area is realized where these larger cluster compounds can be described as an agglomeration of smaller cluster units and might be described as a cluster of clusters.8,9 Thus, the calculations indicate that compact structures become favourable only at a very large cluster size of about 309 tin atoms. Hence, in the case of smaller clusters, a cluster-of-clusters arrangement with principal Sn15 units is energetically favourable. These calculations are supported by gas phase investigations, where naked tin clusters with up to 25 tin atoms might also be described as cluster of clusters with principal Sn9 and Sn10 units.8 This cluster-of-clusters growth pattern seems to be valid up to a certain size of nanoparticles, as observed by Uhlig and co-workers. They observed that spherical nanoparticles are composed of smaller spheres and thus might be seen as particle of particles.10 In the following sections, we will provide an overview of the chemistry of metalloid tin clusters and highlight their potential use as ligands or building blocks to generate larger and more complex structures.Synthesis via reductive coupling
The most straightforward synthesis strategy for the formation of metalloid tin cluster compounds is via the reductive coupling or reductive elimination of tin-containing precursors (Scheme 1). Thereby, even metalloid clusters might be obtained if the complete elimination of substituents takes place during the reduction.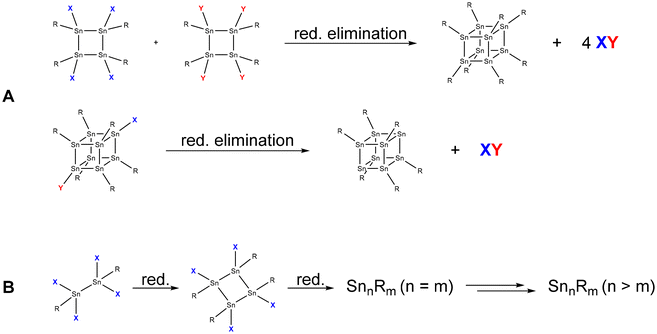 | ||
| Scheme 1 Exemplary formation of tin cluster compounds via reductive elimination (A) and reductive coupling (B). | ||
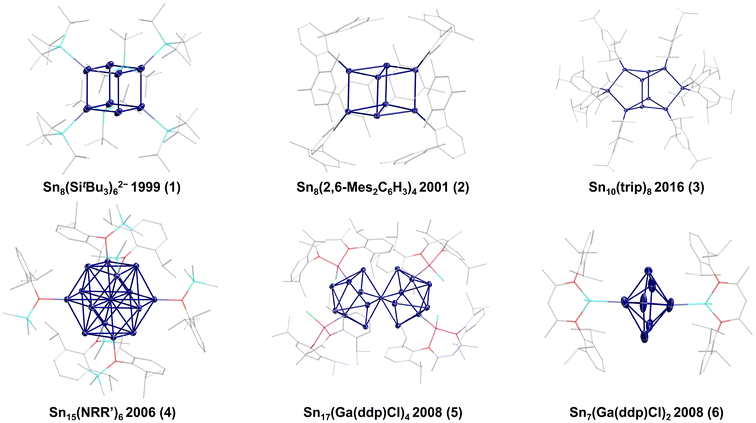
Following this synthetic approach, Wiberg and co-workers were able to synthesize the first metalloid tin cluster [Sn8(SitBu3)6]2−1 in 1999 (Fig. 1).11 Wiberg's [Sn8(SitBu3)6]2− cluster shows a nearly perfect cubic arrangement of the eight tin atoms with minimal distortion (Sn–Sn–Sn angles from 88.06(3) to 91.62(3)°). Hence, the bonding of an SnR or Sn− unit seems to be similar, also showing the relation to the fully saturated clusters Sn8R8 (R = SitBu2Me12 or 2,6-diethylphenyl6), which also show a cubic arrangement of the eight tin atoms.
However, the cubic arrangement of eight group 14 atoms within a group 14 E8Rx cluster depends on the number and kind of substituents present. This is directly obvious within the metalloid Ge8R6 clusters (R = N(SiMe3)2;13 2,6-(tBuO)2-C6H3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14), which show a distorted or an ideal cubic arrangement depending on the kind of ligand used. Also, the number of substituents plays a crucial role, as shown by Power and co-workers for the metalloid Sn8R4 cluster 2 (R = 2,6-Mes2C6H3; Mes = 2,4,6-Me3C6H2), in which the cubic Sn8 core is strongly distorted (Sn–Sn–Sn angles range from 56.24(4) to 112.38(5)°).15 This distortion might be due to the substitution pattern and the high steric demand of the substituents. Nevertheless, the naked tin atoms in 2 are still available for subsequent reactions, thereby leading to the decomposition of the cluster. Hence, the reaction of 2 with hydrogen gives the saturated four-membered ring compound (SnHR)4 and elemental tin. This first example already shows that such metalloid cluster compounds can react with small molecules, thereby also giving an insight into heterogeneous catalytic processes.16 In the subsequent years, only a few additional examples of metalloid tin clusters obtained via reductive coupling have been reported. In 2016, the group of Schulz and co-workers synthesized Sn10(trip)8
14), which show a distorted or an ideal cubic arrangement depending on the kind of ligand used. Also, the number of substituents plays a crucial role, as shown by Power and co-workers for the metalloid Sn8R4 cluster 2 (R = 2,6-Mes2C6H3; Mes = 2,4,6-Me3C6H2), in which the cubic Sn8 core is strongly distorted (Sn–Sn–Sn angles range from 56.24(4) to 112.38(5)°).15 This distortion might be due to the substitution pattern and the high steric demand of the substituents. Nevertheless, the naked tin atoms in 2 are still available for subsequent reactions, thereby leading to the decomposition of the cluster. Hence, the reaction of 2 with hydrogen gives the saturated four-membered ring compound (SnHR)4 and elemental tin. This first example already shows that such metalloid cluster compounds can react with small molecules, thereby also giving an insight into heterogeneous catalytic processes.16 In the subsequent years, only a few additional examples of metalloid tin clusters obtained via reductive coupling have been reported. In 2016, the group of Schulz and co-workers synthesized Sn10(trip)8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 173 (trip = 2,4,6-iPr3C6H2) while Power and co-workers synthesized the Sn15 cluster Sn15R64 (R = N(2,6-iPr2C6H3)(SiMe2Ph)), which exhibits a body-centred, cubic-like arrangement of the tin atoms in the cluster core. This arrangement is realized for tin only in a high-pressure modification above 45 ± 5 GPa.18 As 4 is synthesized at normal pressure and room temperature, this shows that within the metalloid clusters, arrangements can be realized that are obtained for the element only at high pressure. Similar results are also observed within metalloid gallium19 or germanium20 clusters. The largest metalloid tin cluster via reductive coupling was presented by Fischer and co-workers, who were able to synthesize a Sn17 cluster Sn17R45 (R = Ga(ddp)Cl; ddp = HC(CMeNC6H3-2,6-iPr2)2), which is crystallized together with the metalloid cluster Sn7R26, bearing the same substituent.21 The Sn17 cluster 5 can be described as two merged Sn9 subunits, which already shows that larger clusters might be seen as cluster of clusters, where we will see more examples in the following. Anionic Sn9 clusters are also known from Zintl-Ion chemistry, where the structures often nicely fit Wades rules22,23 and which are summarized in recent reviews.24,25 However, Zintl-Ions will not be included in the following as the focus of the perspective is on metalloid clusters, exhibiting an average positive oxidation state of the tin atoms in contrast to Zintl-Ions exhibiting an average negative oxidation state.26
173 (trip = 2,4,6-iPr3C6H2) while Power and co-workers synthesized the Sn15 cluster Sn15R64 (R = N(2,6-iPr2C6H3)(SiMe2Ph)), which exhibits a body-centred, cubic-like arrangement of the tin atoms in the cluster core. This arrangement is realized for tin only in a high-pressure modification above 45 ± 5 GPa.18 As 4 is synthesized at normal pressure and room temperature, this shows that within the metalloid clusters, arrangements can be realized that are obtained for the element only at high pressure. Similar results are also observed within metalloid gallium19 or germanium20 clusters. The largest metalloid tin cluster via reductive coupling was presented by Fischer and co-workers, who were able to synthesize a Sn17 cluster Sn17R45 (R = Ga(ddp)Cl; ddp = HC(CMeNC6H3-2,6-iPr2)2), which is crystallized together with the metalloid cluster Sn7R26, bearing the same substituent.21 The Sn17 cluster 5 can be described as two merged Sn9 subunits, which already shows that larger clusters might be seen as cluster of clusters, where we will see more examples in the following. Anionic Sn9 clusters are also known from Zintl-Ion chemistry, where the structures often nicely fit Wades rules22,23 and which are summarized in recent reviews.24,25 However, Zintl-Ions will not be included in the following as the focus of the perspective is on metalloid clusters, exhibiting an average positive oxidation state of the tin atoms in contrast to Zintl-Ions exhibiting an average negative oxidation state.26
Synthesis via disproportionation reaction
Beside reductive coupling, another approach to metalloid clusters can be seen in the disproportionation reaction of useful precursors. The disproportionation reaction should take place at low temperatures so that the intermediary formed metalloid clusters can be trapped by kinetic stabilization. In case of tin, metastable monohalide solutions, obtained via a preparative co-condensation technique, seem feasible,27,28 following the reaction scheme outlined in Scheme 2.Hence, the isolated clusters are not only intermediates on the way to the element with respect to the average oxidation state of the tin atoms, but also with respect to the synthetic route as without the substitution of the halide atoms by bulky ligands, the disproportionation reaction directly gives elemental tin (Scheme 3).
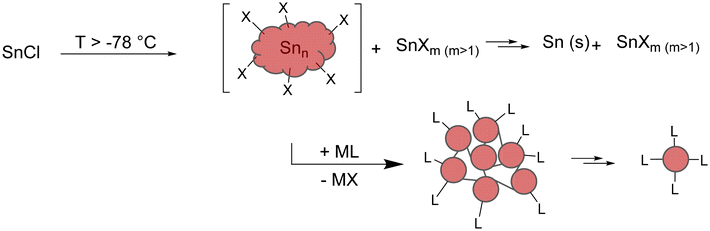 | ||
| Scheme 3 Schematic visualization of the raspberry model. Formation of smallest Sn10-units (e.g., [Sn10(Hyp)4]2−) through the degradation of larger clusters. | ||
Additionally, calculations indicate that in the size range up to 309 tin atoms, structures that can be described as cluster of clusters are energetically favourable, which leads to the general reaction scheme outlined in Scheme 3. Hence, the intermediary formed clusters can be seen as a cluster-of-clusters arrangement, where the principal units are bound together by tin–tin bonds. These primary formed clusters then might be stable and can be isolated, or they can fall apart, leading to smaller clusters with an open substituent shell. Consequently, the bonding between the principal units in the larger clusters is of central importance if larger or smaller metalloid clusters are obtained.
This general idea (raspberry model) about the formation mechanism is supported by the reaction of a metastable Sn(I)Cl solution with LiHyp (Hyp = Si(SiMe3)3), which gives after work-up the metalloid tin cluster [Sn10(Hyp)4]2−7 (Fig. 2)29 in remarkable 33% isolated crystalline yield.
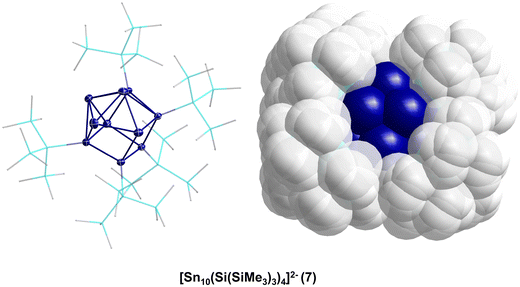
Thereby, the substituent shell is quite open as the four Hyp substituents shield the core of ten tin atoms only marginally (Fig. 2, right). In solution, 7 is stable for a couple of days if Li+ is coordinated by (12)-crown-4. However, if 7 is transferred to the gas-phase via electrospray ionization, [Hyp]− or [SnHyp]− is eliminated, leading to [Sn10(Hyp)3]−830 or [Sn9(Hyp)3]2−9, respectively. Both compounds are also obtained directly applying the disproportionation reaction, indicating that a complex reaction mixture is obtained first, from which, depending on the work-up of the reaction solution, different metalloid clusters are obtained (Fig. 3). Beside the already mentioned clusters, the following clusters are available: [Sn9(Hyp)3]−10,31 [Sn9(Hyp)2]2−11,32 [Sn10(Si(SiMe3)2(Si(SiMe3)3)4)]2−12,33 [Sn10(Si(SiMe3)2(SitBuMe2))4]2−13,34 [Sn10(Ge(SiMe3)3)4]2−14,35 [Sn10(R)5]− (R = Hyp 15,36 Ge(SiMe3)31635), and Sn10(R)6 (R = Hyp 17,37 Ge(SiMe3)31835).
Within all the metalloid tin clusters exhibiting 10 tin atoms (7, 8, 12–18) the arrangement of tin atoms can be described as a more or less distorted centaur polyhedral arrangement, where the tin–tin distances within the icosahedral part are longer and comparable to the distances found in β-tin. The distances in the cubic part are shorter and comparable to the distance found in α-tin. These different kinds of tin atoms are also observed within the Mößbauer spectra, supporting the idea that a phase transition takes place on going from the cubic to the icosahedral part of the cluster. In case of 17, the tin core is well shielded by the six Hyp substituents so that further reactions might not be possible. However, in case of clusters 7–16, the nine or ten tin atoms are only partially shielded by the bulky substituents so that subsequent reactions might be possible.
Such subsequent reactions are well documented for the metalloid germanium clusters [Ge9(Hyp)3]− (ref. 38 and 39) and [Ge9(Hyp)2]2−,40 where oxidative coupling was also realized to give neutral clusters of the composition [Ge18(Hyp)6].41,42
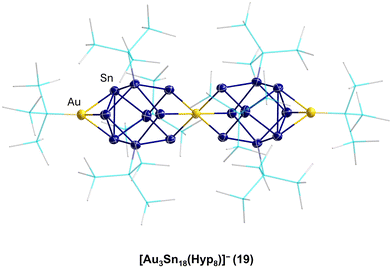
That such subsequent reactions of open shell metalloid tin clusters are also possible was shown in 2017 for [Sn10(Hyp)4]2−7. Thereby, the reaction of 7 with (Ph3P)Au(I)SHyp gives the cluster compound [Au3Sn18(Hyp)8]−19.43 Within 19, two Sn9(Hyp)3 units are bound together by a central Au atom and capped on the other side with an Au(Hyp) unit (Fig. 4).
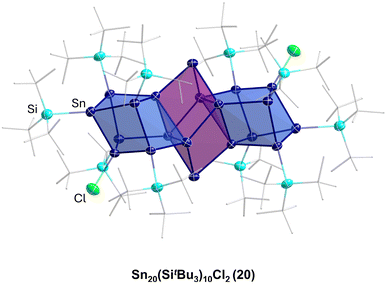
This result directly shows that with respect to germanium, in the case of tin, a more complex reaction system is present as not only can a coordination or a salt metathesis reaction take place but the cluster itself can also decompose, leading to Sn9(Hyp)3 units in this case. Another strong difference with the chemistry of the open shell metalloid germanium clusters is the fact that product 19 is also quite sensitive and decomposes fast in solution to unknown products. In contrast to this, the structurally comparable compound PtZn2Ge18(Hyp)8 is stable in solution.44 The higher sensitivity and dynamic of the metalloid tin clusters with respect to the metalloid germanium clusters might be due to the weaker tin–tin bonds with respect to germanium–germanium bonds. The higher metallic character of tin may also be another factor so that in case of tin, higher coordination numbers are easily realized. To date, mostly hypersilyl-based substituents are used for the synthesis. However, it is known in cluster chemistry that the substituent applied also has an influence on the reactivity and stability of the obtained compound. Consequently, also other substituents were used within the synthesis via the disproportionation reaction, for example, the sterically more demanding substituent SitBu3, leading to Sn20(SitBu3)10Cl220, where not all the halide atoms are substituted and which shows a unique arrangement of the tin atoms, further supporting the raspberry model discussed above.45
Cluster of clusters
The Sn17 cluster 5, synthesized by Fischer et al., might already be described as a cluster built up of two principal Sn9 clusters fused together by one tin atom, belonging to both Sn9 units. The Sn9 framework is thereby well known from metalloid tin clusters or from various Zintl-phases24,25,46 and can therefore be considered as the “smallest” tin-unit in 5. Consequently, the structure of 5 directly provides evidence that larger clusters might be composed of smaller units, as described in the cluster-of-clusters model. While the use of the bulky Si(SiMe3)3 substituents within the synthetic route via the disproportionation reaction almost exclusively leads to Sn10 or Sn9 clusters, the use of the bulky ligand SitBu3 leads to a different compound. Hence, this time, another Sn9 or Sn10 cluster with an open substituent shell is not realized but the metalloid cluster Sn20(SitBu3)10Cl220 with 20 tin atoms is obtained. 20 is the largest structurally characterized metalloid group 14 cluster and is obtained via the disproportionation route using an SnCl solution and NaSitBu3 as the substituent source.45The structure of 20 is remarkable as it can be divided into three subunits (Fig. 5). In the centre, there is an Sn8 unit of naked tin atoms, which exhibits a distorted cubic arrangement (purple polyhedra, Fig. 5). The cubic arrangement thereby resembles the one found in the metalloid cluster Sn8R42 (R = 2,6-Mes2C6H3; Mes = 2,4,6-Me3C6H2)15 with comparable tin–tin distances. To this central Sn8 unit, two Sn6R5Cl units are bound via tin–tin single bonds in the range of 282.3(2)–288.20(19) pm (blue polyhedra, Fig. 5). Within the Sn6R5Cl unit, every tin atom bears a substituent and exhibits the coordination number four, leading to a classical bonding as within the prismatic clusters Sn6R6 (R = SitBu3).47 The Sn20 cluster 20 thus strongly supports the aspect that cluster growth in the nanometre range takes place via cluster of clusters intermediates and depending on the substituent, they might be isolated or decomposed to smaller principal units.
This idea is further supported by calculations focussing on the decomposition of 20 into two Sn10 clusters as these are the ones frequently obtained when Si(SiMe3)3 is used as the ligand source. The structural relation of 20 and a metalloid Sn10 cluster becomes obvious on moving the central tin atoms, as outlined in Scheme 4.
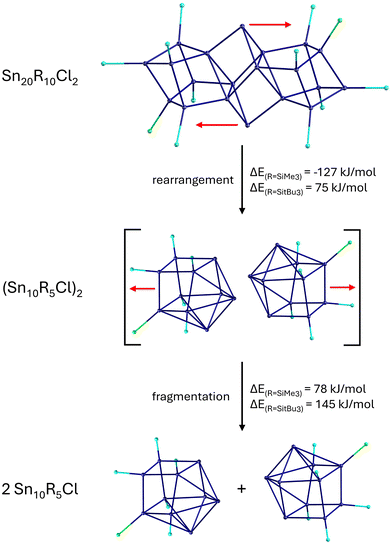 | ||
| Scheme 4 Possible decomposition pathway of 20via rearrangement and fragmentation. Energies calculated for two substituents (R = SiMe3, SitBu3). | ||
After this movement, two loosely bound Sn10R5Cl clusters are realized, which then might dissociate to give smaller less shielded clusters. The movement of the two tin atoms is endothermic in case of the used SitBu3 substituents. However, if the much smaller SiMe3 substituent is used for the calculations, the movement of the tin atoms becomes exothermic, directly showing that the substituent applied has a strong influence if the cluster-of-clusters arrangement or the decomposition to smaller principal units is energetically more favourable. Consequently, to get access to larger metalloid clusters, the choice of the substituent is of central importance.
Conclusion and future outlook
Metalloid tin clusters are ideal atomically precise model compounds for the nanoscaled area, showing novel structures and bonding properties. The naked tin atoms are ideal positions for further reactions, which may also be reversible ones for catalytic applications. However, the subsequent reactions lead up to now always to a decomposition of the primary products. In the area of larger clusters, a cluster of clusters growth seems obvious and thus to get stable larger metalloid tin clusters, useful substituents are needed. Multidentate substituents might be useful to stabilize the intermediary formed larger clusters as these substituents might be able to stabilize the cluster-of-clusters state so that further insight into the fascinating process of cluster growth on the way to the element is possible. However, if the cluster size increases more, the transition area from the cluster of clusters to compact structures would be another interesting area, which might be reached a bit earlier in the case of substituent-stabilized clusters than the naked cluster used during the calculations. The route via disproportionation seems to be the most useful as it intrinsically leads to elemental tin, but we need to find the right reaction conditions and substituents to stop this route in the nanometre range.Author contributions
Roman Kimmich – conceptualization, visualization, writing – original draft, and writing – review and editing. Andreas Schnepf – conceptualization, funding acquisition, project administration, validation, resources, supervision, and writing – review and editing.Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr Claudio Schrenk for helpful discussions and the German Research Foundation (DFG) for financial support through grant no. SCHN738/12-1.References
- L. R. Sita and R. D. Bickerstaff, J. Am. Chem. Soc., 1989, 111, 6454–6456 CrossRef CAS.
- L. R. Sita and I. Kinoshita, J. Am. Chem. Soc., 1992, 114, 7024–7029 CrossRef CAS.
- Structurally related compounds like bicyclo[2.2.1]heptastannane- 1,4-diide and bicyclo[2.2.2]octastannane-1,4-diide have been lately presented by Liptrop and Fischer, (Chem. Commun., 2020, 56, 336).
- L. R. Sita, Acc. Chem. Res., 1994, 27, 191–197 CrossRef CAS.
- L. R. Sita and R. D. Bickerstaff, J. Am. Chem. Soc., 1989, 111, 3769–3770 CrossRef CAS.
- L. R. Sita and I. Kinoshita, Organometallics, 1990, 9, 2865–2867 CrossRef CAS.
- A. Schnepf and H. Schnöckel, Angew. Chem., Int. Ed., 2002, 41, 3532–3554 CrossRef CAS PubMed.
- A. Lechtken, N. Drebov, R. Ahlrichs, M. M. Kappes and D. Schooss, J. Chem. Phys., 2010, 132, 211102 CrossRef PubMed.
- H. Li, W. Chen, F. Wang, Q. Sun, Z. X. Guo and Y. Jia, Phys. Chem. Chem. Phys., 2013, 15, 1831–1836 RSC.
- Results of a PhD-thesis (C. Zeppek, 2015) and a master thesis (S. Reischauer, 2018) prepared in the Uhlig group at TU Graz.
- N. Wiberg, H.-W. Lerner, S. Wagner, H. Nöth and T. Seifert, Z. Naturforsch., B: J. Chem. Sci., 1999, 54, 877–880 CrossRef CAS.
- R. Bashkurov, Y. Kratish, N. Fridman, D. Bravo-Zhivotovskii and Y. Apeloig, Angew. Chem., Int. Ed., 2021, 60, 2898–2902 CrossRef CAS PubMed.
- A. Schnepf and R. Köppe, Angew. Chem., Int. Ed., 2003, 42, 911–913 CrossRef CAS PubMed.
- A. Schnepf and C. Drost, Dalton Trans., 2005, 3277–3280 RSC.
- B. E. Eichler and P. P. Power, Angew. Chem., Int. Ed., 2001, 40, 796–797 CrossRef CAS PubMed.
- N. Y. Tashkandi, E. E. Cook, J. L. Bourque and K. M. Baines, Chem. – Eur. J., 2016, 22, 14006–14012 CrossRef CAS PubMed.
- J. Wiederkehr, C. Wölper and S. Schulz, Chem. Commun., 2016, 52, 12282–12285 RSC.
- M. Brynda, R. Herber, P. B. Hitchcock, M. F. Lappert, I. Nowik, P. P. Power, A. V. Protchenko, A. Růžička and J. Steiner, Angew. Chem., Int. Ed., 2006, 45, 4333–4337 CrossRef CAS PubMed.
- A. Rodig and G. Linti, Angew. Chem., Int. Ed., 2000, 39, 2952–2954 CrossRef CAS PubMed.
- C. Schenk, F. Henke and A. Schnepf, Angew. Chem., Int. Ed., 2013, 52, 1834–1838 CrossRef CAS PubMed.
- G. Prabusankar, A. Kempter, C. Gemel, M. K. Schröter and R. A. Fischer, Angew. Chem., Int. Ed., 2008, 47, 7234–7237 CrossRef CAS PubMed.
- K. Wade, J. Chem. Soc. D, 1971, 15, 792–793 RSC.
- D. M. P. Mingos, Nat. Phys., 1972, 236, 99–102 CrossRef CAS.
- S. Scharfe, F. Kraus, S. Stegmaier, A. Schier and T. F. Fässler, Angew. Chem., Int. Ed., 2011, 50, 3630–3670 CrossRef CAS PubMed.
- R. J. Wilson, B. Weinert and S. Dehnen, Dalton Trans., 2018, 47, 14861–14869 RSC.
- A. Schnepf, in Clusters – Contemporary Insight in Structure and Bonding, ed. S. Dehnen, Springer International Publishing, Chem, 2017, pp. 135–200 Search PubMed.
- C. Schrenk, R. Köppe, I. Schellenberg, R. Pöttgen and A. Schnepf, Z. Anorg. Allg. Chem., 2009, 635, 1541–1548 CrossRef CAS.
- M. Binder, C. Schrenk and A. Schnepf, J. Visualized Exp., 2016, 117, e54498 Search PubMed.
- C. Schrenk, F. Winter, R. Pöttgen and A. Schnepf, Chem. – Eur. J., 2015, 21, 2992–2997 CrossRef CAS PubMed.
- C. Schrenk, B. Gerke, R. Pöttgen, A. Clayborne and A. Schnepf, Chem. – Eur. J., 2015, 21, 8222–8228 CrossRef CAS PubMed.
- C. Schrenk, M. Neumaier and A. Schnepf, Inorg. Chem., 2012, 51, 3989–3995 CrossRef CAS PubMed.
- C. Schrenk, F. Winter, R. Pöttgen and A. Schnepf, Inorg. Chem., 2012, 51, 8583–8588 CrossRef CAS PubMed.
- C. Schrenk and A. Schnepf, Main Group Met. Chem., 2013, 36, 161–167 CAS.
- R. Kimmich, C. Schrenk and A. Schnepf, Dalton Trans., 2021, 50, 16013–16020 RSC.
- M. Binder, C. Schrenk, T. Block, R. Pöttgen and A. Schnepf, Molecules, 2018, 23, 1022 CrossRef PubMed.
- C. Schrenk, J. Helmlinger and A. Schnepf, Z. Anorg. Allg. Chem., 2012, 638, 589–593 CrossRef CAS.
- C. Schrenk, I. Schellenberg, R. Pöttgen and A. Schnepf, Dalton Trans., 2010, 39, 1872–1876 RSC.
- F. Henke, C. Schenk and A. Schnepf, Dalton Trans., 2009, 9141–9145 RSC.
- C. Schenk and A. Schnepf, Angew. Chem., Int. Ed., 2007, 46, 5314–5316 CrossRef CAS PubMed.
- F. S. Geitner and T. F. Fässler, Inorg. Chem., 2020, 59, 15218–15227 CrossRef CAS PubMed.
- O. Kysliak, C. Schrenk and A. Schnepf, Angew. Chem., Int. Ed., 2016, 55, 3216–3219 CrossRef CAS PubMed.
- S. V. Klementyeva, C. Schrenk and A. Schnepf, Inorg. Chem., 2022, 61, 11787–11795 CrossRef CAS PubMed.
- M. Binder, C. Schrenk, T. Block, R. Pöttgen and A. Schnepf, Chem. Commun., 2017, 53, 11314–11317 RSC.
- O. Kysliak, D. D. Nguyen, A. Z. Clayborne and A. Schnepf, Inorg. Chem., 2018, 57, 12603–12609 CrossRef CAS PubMed.
- M. Binder, C. Schrenk and A. Schnepf, Chem. Commun., 2019, 55, 12148–12151 RSC.
- H. G. Von Schnering, M. Baitinger, U. Bolle, W. Carrillo-Cabrera, J. Curda, Y. Grin, F. Heinemann, J. Llanos, K. Peters, A. Schmeding and M. Somer, Z. Anorg. Allg. Chem., 1997, 623, 1037–1039 CrossRef CAS.
- N. Wiberg, H. W. Lerner, H. Nöth and W. Ponikwar, Angew. Chem., Int. Ed., 1999, 38, 1103–1105 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |