Open Access Article
Open Access ArticleIntegrated electrosynthesis and biosynthesis for the production of adipic acid from lignin-derived phenols†
Micjel Chávez
Morejón‡
a,
Alexander
Franz‡
b,
Rohan
Karande
*b and
Falk
Harnisch
 *a
*a
aDepartment of Environmental Microbiology, Helmholtz-Centre for Environmental Research GmbH – UFZ, Permoserstrasse 15, 04318 Leipzig, Germany. E-mail: falk.harnisch@ufz.de
bInterfaculty Centre for Bioactive Matter b-ACT Matter, Leipzig University, Johannisallee 21-23, 04103 Leipzig, Germany. E-mail: rohan.karande@uni-leipzig.de
First published on 8th June 2023
Abstract
We show that adipic acid (AA), a major building block for polyamides like Nylon-6,6, can be synthesized from lignin-derived feedstock by combining electrochemical hydrogenation with biotransformation. Up to 68% yield of cyclohexanol from phenol at 69% coulombic efficiency is achieved for electrochemical hydrogenation, and further, the electrochemical hydrogenation of an aromatic mixture as gained from lignin depolymerization allows 83% yield. Subsequent biotransformation of cyclohexanol with recombinant Pseudomonas taiwanensis VLB120 gains AA at a rate of 0.02 g L−1 h−1 with a yield of up to 61% in 5 h.
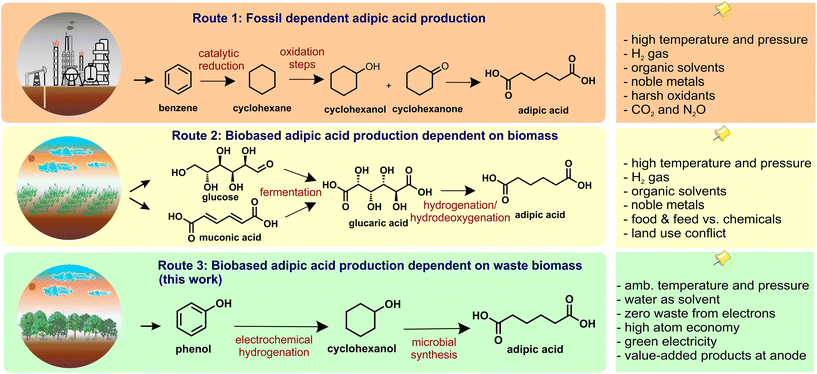
Polyamides (nylons) and polyesters constitute 80% of the worldwide production of synthetic fibers.1 Nylon-6 and Nylon-6,6 – with the latter being based on 50 mol% adipic acid (AA) as a building block – comprise 95% of the total polyamide market with broad applications in automotive engineering, consumer products, and packaging industries.1 In 2021, the AA market was circa US$ 8.1 billion in terms of revenue, exhibiting a compound annual growth rate (CAGR) of 4.9% during the 2022–2030 forecast period.2 The International Energy Agency (IEA) referred to AA as being considered the most important dicarboxylic acid, potentially becoming a platform chemical for bio-based production.3
The current raw materials for industrial AA production, such as cyclohexanol and cyclohexanone, are derived from fossil sources.4 This petrobased synthesis is estimated to be responsible for about 10% of the worldwide anthropogenic N2O.5 Hence, replacing the current petrochemical-based production of AA with an alternative sustainable process will significantly reduce greenhouse gas emissions.6,7 Three main approaches can be distinguished for using bio-based feedstocks for the production of AA: (i) chemo-catalytic processes using glucose;7,8 (ii) indirect fermentation to produce intermediaries (e.g., muconic or glucaric acid) that subsequently undergo chemical dehydrogenation to form AA;9 and (iii) direct microbial fermentation into AA.4,10–18 These approaches might require high temperature and pressure, organic solvents, chemical oxidants, hydrogen or a combination thereof. Furthermore, societal conflicts might need to be addressed when using certain biobased raw materials that require extensive land use (i.e. chemicals vs. food and feed debate (Fig. 1)).
Recently, the microbial conversion of cycloalkanes to monomers such as ε-caprolactone (CL), AA, and 6-aminocaproic acid (6AHA) has been reported under ambient conditions.19–23 For example, a mixed-species approach consisting of recombinant Pseudomonas taiwanensis VLB120 to convert cyclohexane to CL with recombinant Escherichia coli JM101 for further conversion of CL to 6AHA was demonstrated.20 A recombinant P. taiwanensis VLB120 strain synthesizing AA from cyclohexane was also successfully developed.21 However, in all these cases, the substrate for microbial conversion must still be derived from fossil sources. To overcome this limitation, in this study we provide a proof-of-concept for AA production from the renewable waste material lignin using a two-stage process. Liquid fractions from lignin depolymerization are rich in monomeric and oligomeric phenols (e.g., phenol, catechol, syringol, and guaiacol).24 In stage 1, these unsaturated compounds are electrochemically hydrogenated at ambient temperature and no overpressure into their corresponding aliphatic counterparts, i.e., substituted cyclohexane derivatives, making the step environmentally benign.25,26 In stage 2, these cyclohexane derivatives are further converted into monomers such as CL, or AA-like compounds using microbial biotransformation.
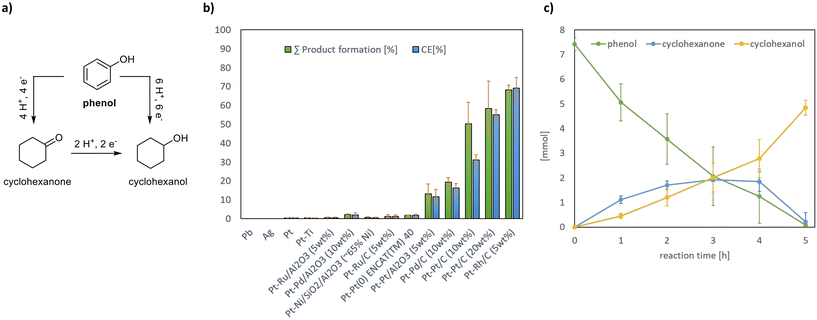
For the proof-of-concept, phenol was used as a model substrate as it represents around 28% of liquid fractions of fully depolymerized lignin.27 Phenol can be reduced directly to cyclohexanol or stepwise through the formation of cyclohexanone as an intermediate (Fig. 2a).
For stage 1, electrochemical hydrogenation of phenol was carried out under potentiostatic control using a two-chamber electrochemical cell in a three-electrode setup consisting of a working electrode (i.e. cathode) set at −1.6 V vs. an Ag/AgCl sat. KCl reference electrode and a counter electrode (Pt). For the electrochemical hydrogenation of phenol, extensive screening of four monolithic cathodes and ten suspended heterogeneous catalysts combined with monolithic cathodes was performed in phosphate buffer as a supporting electrolyte, making the electrochemical conversion compatible with the later biotransformation. In brief, the heterogeneous catalysts combined with monolithic cathodes showed better performance (Fig. 2b). The utilization of catalysts supported on carbon has recently been described for the electrochemical-mediated hydrogenation of aromatic compounds under different conditions and with varying efficiencies.28–32
The study of combined monolithic cathodes with suspended catalysts in 1 M KH2PO4/K2HPO4 (Fig. 2b) allowed the identification of three promising candidates, with Rh/C (5 wt%) yielding the highest product formation (68%) at 69% coulombic efficiency (CE). The utilization of Pt/C (10 wt%) allowed for 50% product formation at 31% CE and a mass balance of 100%, while Pt/C (20 wt%) yielded 58% product formation at 55% CE with a mass balance of 75% (Fig. S2†).
The time-resolved analysis confirmed that during the hydrogenation using a Pt cathode with Rh/C (5 wt%), 100 mM of phenol was fully consumed after 5 h. Interestingly, after 5 h, cyclohexanol was gained with 100% selectivity, as cyclohexanone was detected only as an intermediate. This suggests that cyclohexanone and cyclohexanol are initially produced in parallel, while the formed cyclohexanone undergoes further hydrogenation to cyclohexanol (Fig. 2c).
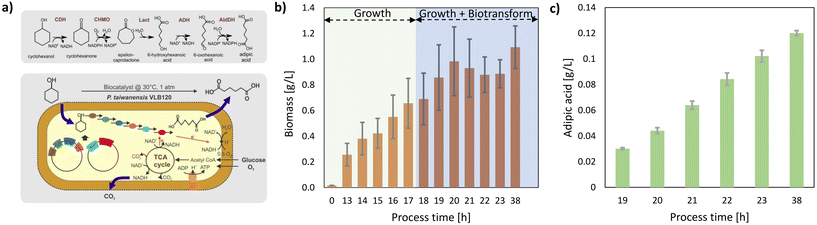
For stage 2, the cyclohexanol derived from the electrochemical hydrogenation of phenol using a Pt cathode with Pt/C (20 wt%) after 22 h of reaction was further valorised into AA using biotransformation by microbial synthesis. Although cyclohexanol is used as a starting compound for the enzyme cascade for AA production by Pseudomonas taiwanensis VLB120 (pSEVA_6HA_2, pBX_AA_Ac) (Fig. 3a),21 it strongly inhibits cyclohexanone monooxygenase (CHMO) with a 50% reduction in activity at a cyclohexanol concentration as low as 0.4 mM.22 Although P. taiwanensis VLB120 showed a maximum activity of 73 U gCDW−1 at 0.2 mM of cyclohexanol, AA accounted for 66% of the total product with cyclohexanone and 6-hydroxyhexanoic acid as the accumulating by-products.22 Therefore, cyclohexanol was continuously added using a fed-batch approach to enable substrate-limited biotransformation to minimise cyclohexanol inhibition and enhance AA production.
P. taiwanensis VLB120 cells were grown in batch mode in a bubble column reactor and were induced at 0.38 gCDW L−1 by adding IPTG and m-toluic acid for heterologous cascade gene expression. After induction, the growth rate of P. taiwanensis was 0.16 h−1. The biotransformation was started after 18 h by continuously feeding cyclohexanol (0.102 mmol cyclohexanol h−1) at a flow rate of 41.3 μL min−1 over 5 h for the production of AA (Fig. 3b). AA was produced at a rate of 0.02 g L−1 h−1 (Fig. 3c) for 5 h with a yield higher than 60% based on the fed cyclohexanol. As the biotransformation was operated under substrate limitation to minimize cyclohexanol inhibition, the average specific activity of 2.5–3 U gCDW−1 was lower than the initial specific activity of 48 U gCDW−1.21 Therefore, a further increase in the cyclohexanol feed rate will enhance the cyclohexanol availability for biotransformation and, subsequently, the AA production rate. Altogether, the combined process yielded 40% for the production of AA based on the starting material phenol. Further improvements are necessary to maximize the combined process yields. For the electrochemical process, for instance, electrosynthesis in a gas-tight setup (to avoid evaporation of the cyclohexane that can be potentially formed), using an optimized potential, as well as avoiding/reducing the transfer of substrates to the anode compartment (see the ESI, section 2, page S5†), might contribute to improving electrochemical yields. This covers all aspects of electrochemical and reactor engineering, like, for instance, the cathode potential/current density, the electrode architecture, and the catalyst as well as the electrode surface to reactor volume ratio.33
The biotransformation yield of 60% on cyclohexanol suggests either the formation of intermediate products such as cyclohexanone and 6-hydroxyhexanoic acid or further degradation of AA by P. taiwanensis. As intermediate compounds were not detected during the biotransformation, the loss of AA might result from product degradation. A previous study suggested that P. taiwanensis is capable of AA degradation at a rate of 0.3 U gCDW−1.34 To overcome product degradation and improve the biotransformation yield, further developments are necessary for in situ product removal using an organic phase or solid phase to extract AA from the reaction mixture.
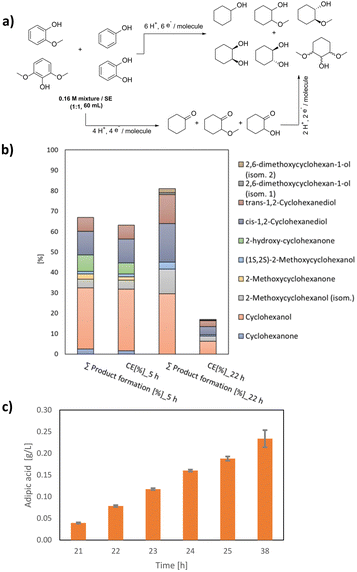
Lignin depolymerization provides a range of aromatic compounds, including monomers like phenol, syringol, catechol and guaiacol.35 Hence, an artificial aromatic mixture that resembles liquid fractions from lignin depolymerization (for further information, see the ESI†) was hydrogenated using Rh/C (5 wt%) as the catalyst, yielding 68% product formation at 73% CE after 5 h of reaction. It is noteworthy that the yield may be underestimated, as losses of substrate towards the anode chamber occurred (see the ESI, section 2, page S5†) and likely too, due to the evaporation of cyclohexane that can be potentially derived from the electrochemical hydrodeoxygenation of the hydroxyl group present in the substrate phenol. As depicted in Fig. 4a, the product distribution is diverse and varies along the reaction time, allowing tailoring of the power-to-chemicals approach to different scenarios. For example, when electric energy is cheap, it is advantageous to perform longer reaction times, increasing the yield of the product to 83%, while shorter reaction times allow a balanced outcome between product formation (65–70% yield) and energy utilization (65–78% CE) (Fig. 4b).
These gained cyclohexane derivatives were further valorised using the above-described microbial synthesis into AA and AA-like compounds. As shown in Fig. 4c, the biotransformation of an electrochemically derived mixture of saturated compounds resulted in a steady production of AA at a rate of 0.038 g L−1 h−1. The formation of other AA-like compounds formed during this biotransformation is currently under investigation. The overall efficiency of the combined route is 57% when the aromatic mixture is electrochemically hydrogenated for 22 h (83% conversion, 24% CE), and the cyclohexane derivatives mixture is biotransformed into AA during 5 h (68% yield).
In the current example, the E-factor is between 5000 and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for AA synthesis from phenol and phenol derivatives, respectively (Table S3†). The E-Factor determines the amount of waste produced compared to the amount of synthesised desired product. A lower E-factor corresponds to a positive environmental impact due to there being less waste. Compared to the recommended E-factor for the bulk chemical production of 1–5,36 the AA synthesis shown here displays several thousand-fold higher E-factor values. However, water is the main contributor to the E-factor, which accounts for almost 96%. Therefore, recycling and reusing water and developing high-cell-density cultivation of Pseudomonas species to obtain high volumetric productivities for future process developments are necessary and likely feasible to minimise waste production for the E-factor.
000 for AA synthesis from phenol and phenol derivatives, respectively (Table S3†). The E-Factor determines the amount of waste produced compared to the amount of synthesised desired product. A lower E-factor corresponds to a positive environmental impact due to there being less waste. Compared to the recommended E-factor for the bulk chemical production of 1–5,36 the AA synthesis shown here displays several thousand-fold higher E-factor values. However, water is the main contributor to the E-factor, which accounts for almost 96%. Therefore, recycling and reusing water and developing high-cell-density cultivation of Pseudomonas species to obtain high volumetric productivities for future process developments are necessary and likely feasible to minimise waste production for the E-factor.
Conclusions
This study demonstrates the production of AA—a key building block for synthesising Nylon-6,6—based on lignin-derived feedstock. The utilization of phenol as a model substrate allowed us to identify Rh/C (5 wt%) as the best catalyst to carry out the electrochemical hydrogenation of the aromatic compounds, with an average yield of about 70% at 66–78% CE depending on the substrate used. The electrochemically produced cyclohexanol was further converted into AA using a five-step enzyme cascade in the strain P. taiwanensis VLB120. The production rate of AA was about 0.02 g L−1 h−1 with an overall combined yield of 40% (see also Table S4†). Moreover, the feasibility of the integrated process was assessed using an artificial aromatic mixture that resembles liquid fractions from lignin depolymerization. The combined route resulted in a steady production of AA with an overall yield of 57% when the aromatic mixture is subjected to electrochemical hydrogenation for longer reaction times (usually 22 h). The results suggest that more than one component of the electrochemically hydrogenated mixture is converted to AA. The electrobiorefinery process outlined here fits well into existing production lines and addresses the industrial demand for a biobased, green, circular economy.Author contributions
MCM: conceptualization, methodology, investigation, validation, visualization, and writing – original draft preparation. AF: conceptualization, methodology, investigation, validation, visualization, and writing – original draft preparation. RK: conceptualization, methodology, resources, funding acquisition, writing – review and editing, and supervision. FH: conceptualization, methodology, resources, funding acquisition, writing – review and editing, and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financed by the UFZ internal programme transfun of the Department of Knowledge and Technology transfer with resources from the Call “Innovation Fund of the Helmholtz Centers” provided by the Joint Initiative for Research and Innovation and supported by the Helmholtz-Association in the frame of the Integration Platform “Tapping nature's potential for sustainable production and a healthy environment” at the UFZ. Furthermore, AF and RK were funded by the Federal Ministry for Economic Affairs and Energy (BMWi, STARK program, project number 46SKD023X) and are co-financed by the Saxon state parliament (Sächsisches Staatsministerium für Wissenschaft, Kultur und Tourismus (SMWK)).References
- G. Bellussi and C. Perego, CATTECH, 2000, 4, 4–16 CrossRef CAS.
- https://www.coherentmarketinsights.com/market-insight/adipic-acid-market-318 .
- E. de Jong, A. Higson, P. Walsh and M. Wellisch, IEA Bioenergy, Task42 Biorefinery, 2012, vol. 34, pp. 1–33 Search PubMed.
- T. Polen, M. Spelberg and M. Bott, J. Biotechnol., 2013, 167, 75–84 CrossRef CAS PubMed.
- S. Alini, F. Basile, S. Blasioli, C. Rinaldi and A. Vaccari, Appl. Catal., B, 2007, 70, 323–329 CrossRef CAS.
- R. Aryapratama and M. Janssen, J. Cleaner Prod., 2017, 164, 434–443 CrossRef CAS.
- G. M. Diamond, V. Murphy and T. R. Boussie, Mod Appl high throughput RD Heterog Catal, 2014, pp. 288–309 Search PubMed.
- E. L. D. Thomas, R. Boussie, Z. M. Fresco and V. J. Murphy, Production of Adipic Acid and Derivatives from Carbohydate-Containing Materials, US-8501989-B2, 2013.
- C. Weber, C. Bruckner, S. Weinreb, C. Lehr, C. Essl and E. Boles, Appl. Environ. Microbiol., 2012, 78, 8421–8430 CrossRef CAS PubMed.
- T. Beardslee and S. Picataggio, Lipid Technol., 2012, 24, 223–225 CrossRef CAS.
- T. Babu, E. J. Yun, S. Kim, D. H. Kim, K. H. Liu, S. R. Kim and K. H. Kim, Process Biochem., 2015, 50, 2066–2071 CrossRef CAS.
- J. C. J. Bart and S. Cavallaro, Ind. Eng. Chem. Res., 2015, 54, 567–576 CrossRef CAS.
- Y. Deng, L. Z. Ma and Y. Mao, Biochem. Eng. J., 2016, 105, 16–26 CrossRef CAS.
- Y. Deng and Y. Mao, J. Appl. Microbiol., 2015, 119, 1057–1063 CrossRef CAS PubMed.
- N. Kallscheuer, J. Gatgens, M. Lubcke, J. Pietruszka, M. Bott and T. Polen, Appl. Microbiol. Biotechnol., 2017, 101, 2371–2382 CrossRef CAS PubMed.
- N. S. Kruyer and P. Peralta-Yahya, Curr. Opin. Biotechnol, 2017, 45, 136–143 CrossRef CAS PubMed.
- S. C. Turk, W. P. Kloosterman, D. K. Ninaber, K. P. Kolen, J. Knutova, E. Suir, M. Schurmann, P. C. Raemakers-Franken, M. Muller, S. M. de Wildeman, L. M. Raamsdonk, R. van der Pol, L. Wu, M. F. Temudo, R. A. van der Hoeven, M. Akeroyd, R. E. van der Stoel, H. J. Noorman, R. A. Bovenberg and A. C. Trefzer, ACS Synth. Biol., 2016, 5, 65–73 CrossRef CAS PubMed.
- J. L. Yu, X. X. Xia, J. J. Zhong and Z. G. Qian, Biotechnol. Bioeng., 2014, 111, 2580–2586 CrossRef CAS PubMed.
- R. Karande, K. Buhler, D. Salamanca Velandia, K.-L. Engesser and A. Schmid, WO/2018/046104, 2016.
- L. Bretschneider, M. Wegner, K. Buhler, B. Buhler and R. Karande, Microb. Biotechnol., 2021, 14, 1011–1025 CrossRef CAS PubMed.
- L. Bretschneider, I. Heuschkel, K. Buhler, R. Karande and B. Buhler, Metab. Eng., 2022, 70, 206–217 CrossRef CAS PubMed.
- L. Schafer, K. Buhler, R. Karande and B. Buhler, Biotechnol. J., 2020, 15, e2000091 CrossRef PubMed.
- R. Karande, D. Salamanca, A. Schmid and K. Buehler, Biotechnol. Bioeng., 2018, 115, 312–320 CrossRef CAS PubMed.
- N. N. Zhou, W. P. D. W. Thilakarathna, Q. S. He and H. P. V. Rupasinghe, Front. Energy Res., 2022, 9, 758744 CrossRef.
- F. Harnisch and M. C. Morejon, Chem. Rec., 2021, 21, 2277–2289 CrossRef CAS PubMed.
- C. H. Lam, C. B. Lowe, Z. L. Li, K. N. Longe, J. T. Rayburn, M. A. Caldwell, C. E. Houdek, J. B. Maguire, C. M. Saffron, D. J. Miller and J. E. Jackson, Green Chem., 2015, 17, 601–609 RSC.
- S. B. Liang and C. X. Wan, Waste Biomass Valorization, 2017, 8, 393–400 CrossRef CAS.
- Y. P. Wijaya, K. J. Smith, C. S. Kim and E. L. Gyenge, J. Appl. Electrochem., 2021, 51, 51–63 CrossRef CAS.
- Y. P. Wijaya, T. Grossmann-Neuhaeusler, R. D. Dhewangga Putra, K. J. Smith, C. S. Kim and E. L. Gyenge, ChemSusChem, 2020, 13, 629–639 CrossRef CAS PubMed.
- N. Singh, U. Sanyal, G. Ruehl, K. A. Stoerzinger, O. Y. Gutierrez, D. M. Camaioni, J. L. Fulton, J. A. Lercher and C. T. Campbell, J. Catal., 2020, 382, 372–384 CrossRef CAS.
- I. Barth, J. Akinola, J. Lee, O. Y. Gutierrez, U. Sanyal, N. Singh and B. R. Goldsmith, J. Chem. Phys., 2022, 156, 104703 CrossRef CAS PubMed.
- M. Garedew, D. Young-Farhat, J. E. Jackson and C. M. Saffron, ACS Sustainable Chem. Eng., 2019, 7, 8375–8386 CrossRef CAS.
- E. J. Biddinger and M. A. Modestino, Electrochem. Soc. Interface, 2020, 29, 43 CrossRef CAS.
- L. Bretschneider, Doctoral dissertation, Natural Sciences Faculty I - Biosciences - of the Martin Luther University Halle-Wittenberg, 2021.
- R. Katahira, A. Mittal, K. McKinney, X. W. Chen, M. P. Tucker, D. K. Johnson and G. T. Beckham, ACS Sustainable Chem. Eng., 2016, 4, 1474–1486 CrossRef CAS.
- R. A. Sheldon, The E Factor: fifteen years on, Green Chemistry, 2007, 9, 1273–1283 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, liquid phase analysis, and supplementary experiments. See DOI: https://doi.org/10.1039/d3gc01105d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |