Themed collection QSARs and computational chemistry methods in environmental chemical sciences

Front cover

Inside back cover

Back cover

Contents list
QSARs and computational chemistry methods in environmental chemical sciences
Guest editors Kathrin Fenner and Paul Tratnyek introduce the themed issue on “QSARs and computational chemistry methods in environmental chemical sciences” of Environmental Science: Processes & Impacts.
Environ. Sci.: Processes Impacts, 2017,19, 185-187
https://doi.org/10.1039/C7EM90008B
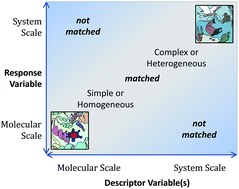
In silico environmental chemical science: properties and processes from statistical and computational modelling
Theoretical and statistical approaches to calculation of properties that determine the environmental fate and effects of substances are summarized, with emphasis on their integration into “in silico environmental chemical science”.
Environ. Sci.: Processes Impacts, 2017,19, 188-202
https://doi.org/10.1039/C7EM00053G
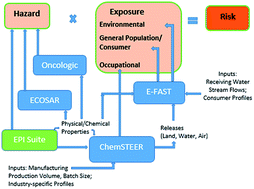
History of EPI Suite™ and future perspectives on chemical property estimation in US Toxic Substances Control Act new chemical risk assessments
A discussion of the past developments, current practices, and future opportunities in QSAR modeling for new chemical risk assessments.
Environ. Sci.: Processes Impacts, 2017,19, 203-212
https://doi.org/10.1039/C7EM00064B
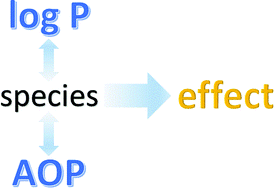
(Q)SARs to predict environmental toxicities: current status and future needs
An assessment of (Q)SARs to predict acute and chronic ecotoxicity.
Environ. Sci.: Processes Impacts, 2017,19, 213-220
https://doi.org/10.1039/C6EM00687F
A review of quantitative structure–property relationships for the fate of ionizable organic chemicals in water matrices and identification of knowledge gaps
QSPR prediction models for chemical fate and exposure are critically reviewed so that knowledge gaps may be filled in subsequent study.
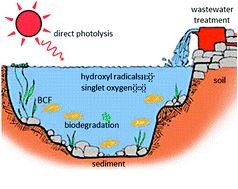
Environ. Sci.: Processes Impacts, 2017,19, 221-246
https://doi.org/10.1039/C7EM00034K
Virulence factor activity relationships (VFARs): a bioinformatics perspective
Virulence factor activity relationships (VFARs) – a concept loosely based on quantitative structure–activity relationships (QSARs) for chemicals was proposed as a predictive tool for ranking risks due to microorganisms relevant to water safety.
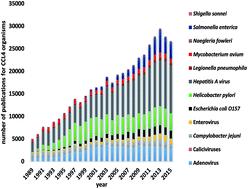
Environ. Sci.: Processes Impacts, 2017,19, 247-260
https://doi.org/10.1039/C6EM00689B
Ranking REACH registered neutral, ionizable and ionic organic chemicals based on their aquatic persistency and mobility
REACH registered neutral, ionizable and ionic organic chemicals were evaluated for their potential to present a hazard to drinking water sources.
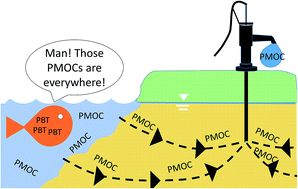
Environ. Sci.: Processes Impacts, 2017,19, 939-955
https://doi.org/10.1039/C7EM00158D
Baseline toxicity and ion-trapping models to describe the pH-dependence of bacterial toxicity of pharmaceuticals
The pH-dependence of cytotoxicity of diverse acidic, basic and multiprotic pharmaceuticals could be explained by baseline toxicity after invoking mixture effects of all species and delayed uptake of charged species.
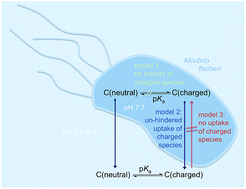
Environ. Sci.: Processes Impacts, 2017,19, 901-916
https://doi.org/10.1039/C7EM00099E
Eawag-Soil in enviPath: a new resource for exploring regulatory pesticide soil biodegradation pathways and half-life data
Eawag-Soil offers an extensive collection of data on pesticide soil degradation pathways and half-lives for diverse uses, including QSBR development.
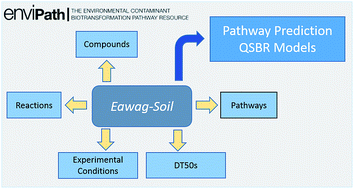
Environ. Sci.: Processes Impacts, 2017,19, 449-464
https://doi.org/10.1039/C6EM00697C
Development of a QSAR model for predicting aqueous reaction rate constants of organic chemicals with hydroxyl radicals
This study provides a QSAR model for predicting the aqueous reaction rate constants of organic chemicals with hydroxyl radicals.
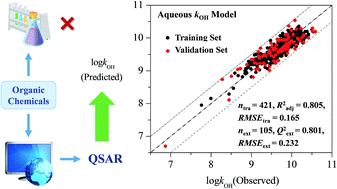
Environ. Sci.: Processes Impacts, 2017,19, 350-356
https://doi.org/10.1039/C6EM00707D
Prediction of acute toxicity of emerging contaminants on the water flea Daphnia magna by Ant Colony Optimization–Support Vector Machine QSTR models
Prediction of acute toxicity towards Daphnia magna using Ant Colony Optimization–Support Vector Machine QSTR models.
Environ. Sci.: Processes Impacts, 2017,19, 438-448
https://doi.org/10.1039/C6EM00679E
General baseline toxicity QSAR for nonpolar, polar and ionisable chemicals and their mixtures in the bioluminescence inhibition assay with Aliivibrio fischeri
A general QSAR model for the Microtox assay with the ionisation-corrected liposome–water distribution ratio is applicable to diverse chemicals including acids and bases.
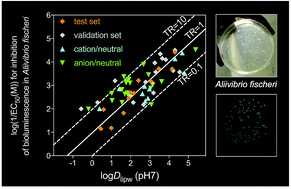
Environ. Sci.: Processes Impacts, 2017,19, 414-428
https://doi.org/10.1039/C6EM00692B
Sulfate radical oxidation of aromatic contaminants: a detailed assessment of density functional theory and high-level quantum chemical methods
DFT and high-level quantum methods are utilized to explore sulfate radical-driven oxidation.
Environ. Sci.: Processes Impacts, 2017,19, 395-404
https://doi.org/10.1039/C7EM00009J
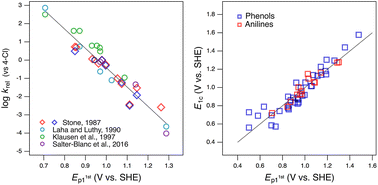
Oxidation potentials of phenols and anilines: correlation analysis of electrochemical and theoretical values
New experimental and theoretical oxidation potentials for substituted phenols and anilines give improved correlations to kinetic data with manganese oxides.
Environ. Sci.: Processes Impacts, 2017,19, 339-349
https://doi.org/10.1039/C6EM00694A
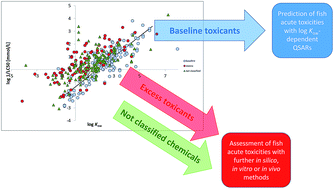
Classification of baseline toxicants for QSAR predictions to replace fish acute toxicity studies
Classification of baseline and excess toxicants to replace 50% of fish acute toxicity testing with reliable QSAR predictions.
Environ. Sci.: Processes Impacts, 2017,19, 429-437
https://doi.org/10.1039/C6EM00600K
Diverging effects of isotopic fractionation upon molecular diffusion of noble gases in water: mechanistic insights through ab initio molecular dynamics simulations
Simulation snapshot of a dissolved krypton atom within a typical water cavity responsible for the size dependent diffusion behavior of noble gases.
Environ. Sci.: Processes Impacts, 2017,19, 405-413
https://doi.org/10.1039/C6EM00614K
The degradation mechanism of sulfamethoxazole under ozonation: a DFT study
Sulfamethoxazole (SMX), a kind of antibiotic, remains in the environment and threatens public health.
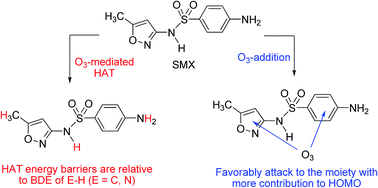
Environ. Sci.: Processes Impacts, 2017,19, 379-387
https://doi.org/10.1039/C6EM00698A
Predicting the phospholipophilicity of monoprotic positively charged amines
The sorption affinity of eighty-six charged amine structures to phospholipid monolayers (log KIAM) was determined using immobilized artificial membrane high-performance liquid chromatography (IAM-HPLC).
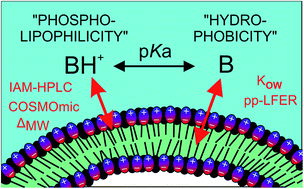
Environ. Sci.: Processes Impacts, 2017,19, 307-323
https://doi.org/10.1039/C6EM00615A
Linear free energy relationships for the adsorption of volatile organic compounds onto multiwalled carbon nanotubes at different relative humidities: comparison with organoclays and activated carbon
Adsorption behavior of volatile organic compounds (VOCs) on carbon nanotubes is critical for developing effective assessment and treatments for nanomaterial-bound contaminants.

Environ. Sci.: Processes Impacts, 2017,19, 276-287
https://doi.org/10.1039/C6EM00567E
Quantifying the equilibrium partitioning of substituted polycyclic aromatic hydrocarbons in aerosols and clouds using COSMOtherm
Functional groups attached to polycyclic aromatic hydrocarbons (PAHs) can significantly modify the environmental fate of the parent compound.
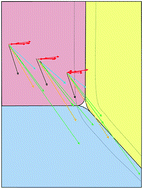
Environ. Sci.: Processes Impacts, 2017,19, 288-299
https://doi.org/10.1039/C6EM00636A
A computer-based prediction platform for the reaction of ozone with organic compounds in aqueous solution: kinetics and mechanisms
A computer-based prediction platform for predicting kinetics and pathways for the reaction of ozone with micropollutants was developed.
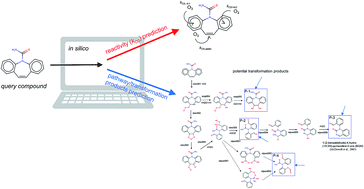
Environ. Sci.: Processes Impacts, 2017,19, 465-476
https://doi.org/10.1039/C6EM00584E
Development of polyparameter linear free energy relationship models for octanol–air partition coefficients of diverse chemicals
This study develops pp-LFER models to predict octanol–air partition coefficients at different temperatures for diverse chemicals.

Environ. Sci.: Processes Impacts, 2017,19, 300-306
https://doi.org/10.1039/C6EM00626D
In silico kinetics of alkaline hydrolysis of 1,3,5-trinitro-1,3,5-triazinane (RDX): M06-2X investigation
The kinetics of alkaline hydrolysis of 1,3,5-trinitro-1,3,5-triazinane were predicted under different conditions.
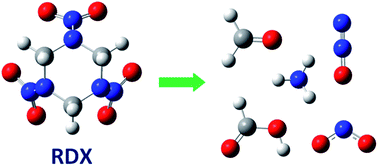
Environ. Sci.: Processes Impacts, 2017,19, 388-394
https://doi.org/10.1039/C6EM00565A
3D-QSAR predictions for bovine serum albumin–water partition coefficients of organic anions using quantum mechanically based descriptors
The 3D-QSAR model predicts the bovine serum albumin–water partition coefficients for neutral and anionic chemicals influenced by steric effects.
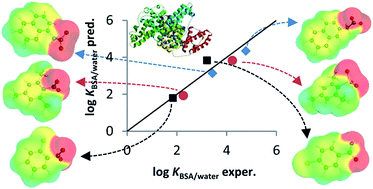
Environ. Sci.: Processes Impacts, 2017,19, 261-269
https://doi.org/10.1039/C6EM00555A
QSARs for phenols and phenolates: oxidation potential as a predictor of reaction rate constants with photochemically produced oxidants
One electron oxidation potential predicts the reactivity of phenol and phenolate compounds in a single correlation.
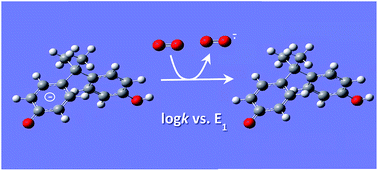
Environ. Sci.: Processes Impacts, 2017,19, 324-338
https://doi.org/10.1039/C6EM00580B
Atmospheric oxidation of halogenated aromatics: comparative analysis of reaction mechanisms and reaction kinetics
This study provides valuable insight into the mechanism of tropospheric degradation and fate of halogenated aromatic systems.

Environ. Sci.: Processes Impacts, 2017,19, 357-369
https://doi.org/10.1039/C6EM00577B
Ferrate(VI) initiated oxidative degradation mechanisms clarified by DFT calculations: a case for sulfamethoxazole
Ferrate(VI) is an efficient and environmentally friendly oxidant for the degradation of organic micropollutants.
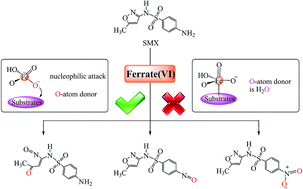
Environ. Sci.: Processes Impacts, 2017,19, 370-378
https://doi.org/10.1039/C6EM00521G
Oligomeric models for estimation of polydimethylsiloxane–water partition ratios with COSMO-RS theory: impact of the combinatorial term on absolute error
PDMS passive sampling media effectively modelled with small oligomers using COSMO-RS.
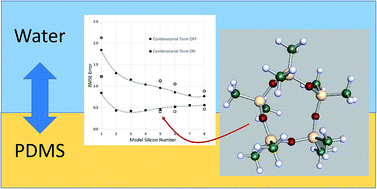
Environ. Sci.: Processes Impacts, 2017,19, 270-275
https://doi.org/10.1039/C6EM00355A
About this collection
Quantitative structure-activity relationships (QSARs) have long been used in environmental sciences. More recently, molecular modeling and chemoinformatic methods have become widespread. These methods have the potential to expand and accelerate advances in environmental chemistry because they complement observational and experimental data with “in silico” results and analysis.
To encourage these advances, the guest editors of this themed issue, Paul Tratnyek (OHSU) and Kathrin Fenner (Eawag), recruited papers from among the best practitioners of in silico environmental science. Authors were selected to represent the diversity of this community, including disciplinary perspectives and intended applications.
The specific topics covered in this issue range from detailed studies of specific processes using advanced molecular methods—such as the diffusion of noble gases in water, or the mechanism of antibiotic oxidation by ozone—to more general applications of statistical and “big data” approaches—such as data mining for new transformation pathways and multi-dimensional metrics for ecological risk assessment.
The resulting collection of perspectives, critical reviews and research papers provides a comprehensive and balanced perspective on the whole range of “in silico” methods in environmental science at this time. Hopefully, it also will encourage and expand the opportunities that computational approaches offer for further advances in environmental chemistry, ecotoxicology, risk assessment, and related aspects of environmental science.