Themed collection Most popular 2018-2019 organic chemistry articles

New avenues for C–B bond formation via radical intermediates
Efficient radical routes to important alkyl and aryl boronic esters have been developed over the past few years. Such reactions are complementary to existing transition-metal catalysed cross coupling processes.
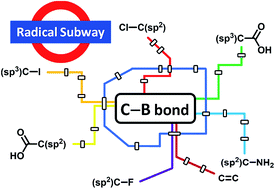
Chem. Sci., 2019,10, 8503-8518
https://doi.org/10.1039/C9SC03765A
A synthetic chemist's guide to electroanalytical tools for studying reaction mechanisms
A range of electroanalytical tools can be applied to studying redox reactions, probing key mechanistic questions in synthetic chemistry.
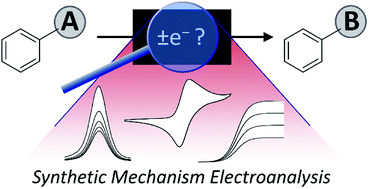
Chem. Sci., 2019,10, 6404-6422
https://doi.org/10.1039/C9SC01545K
C4–H indole functionalisation: precedent and prospects
This Perspective article traces the evolution of modern approaches to functionalise the indole C4–H bond.
Chem. Sci., 2018,9, 4203-4216
https://doi.org/10.1039/C7SC05336C
Mechanochemistry as an emerging tool for molecular synthesis: what can it offer?
Mechanochemistry is becoming more widespread as a technique for molecular synthesis with new mechanochemical reactions being discovered at increasing frequency. This perspective explores what more it can offer, aside from the clear benefit of reduced solvent consumption.

Chem. Sci., 2018,9, 3080-3094
https://doi.org/10.1039/C7SC05371A
Multicomponent mechanochemical synthesis
Multicomponent reactions promoted by mechanical energy are critically reviewed.

Chem. Sci., 2018,9, 2042-2064
https://doi.org/10.1039/C7SC05370C
Tailoring polymer dispersity and shape of molecular weight distributions: methods and applications
This review explores the different synthetic methods by which dispersity and MWD shape can be tuned and discusses the different properties and applications where this variation is beneficial.
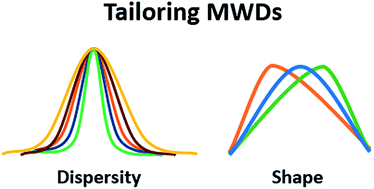
Chem. Sci., 2019,10, 8724-8734
https://doi.org/10.1039/C9SC03546J
Predictive and mechanistic multivariate linear regression models for reaction development
The utilization of physical organic molecular descriptors for the quantitative description of reaction outcomes in multivariate linear regression models is demonstrated as an effective tool for a priori prediction and mechanistic interrogation.
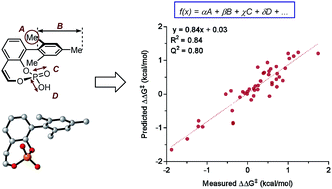
Chem. Sci., 2018,9, 2398-2412
https://doi.org/10.1039/C7SC04679K
sp3 C–H activation via exo-type directing groups
The application of exo-type directing groups (DGs) has led to the discovery of a wide range of novel C(sp3)–H activation methods, which allow efficient and site-selective functionalization of alcohol and amide derivatives.
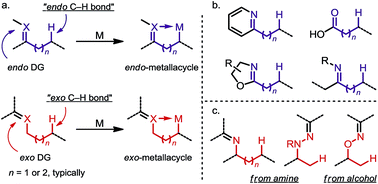
Chem. Sci., 2018,9, 1424-1432
https://doi.org/10.1039/C7SC04768A
Photocatalytic carbanion generation from C–H bonds – reductant free Barbier/Grignard-type reactions
We report a redox-neutral method for the generation of carbanions from benzylic C–H bonds in a photocatalytic Grignard-type reaction.
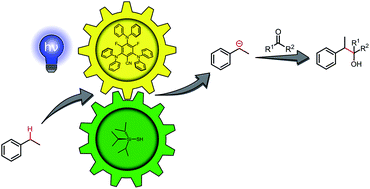
Chem. Sci., 2019,10, 10991-10996
https://doi.org/10.1039/C9SC04987H
Ni-catalyzed 1,2-benzylboration of 1,2-disubstituted unactivated alkenes
A method for the 1,2-benzylboration of unactivated alkenes is presented. The reaction combines readily available alkenes, diboron reagents and benzylchlorides to generate synthetically versatile products with control of stereochemistry.
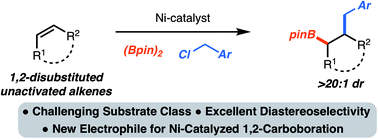
Chem. Sci., 2019,10, 10944-10947
https://doi.org/10.1039/C9SC04199K
Merging hypervalent iodine and sulfoximine chemistry: a new electrophilic trifluoromethylation reagent
Two prominent trifluoromethylation reagent classes join forces in a bench stable hypervalent iodosulfoximine CF3 transfer agent. We report its synthesis, properties and reactivity, opening up new possibilities in trifluoromethylation chemistry.

Chem. Sci., 2019,10, 10516-10523
https://doi.org/10.1039/C9SC04289J
A dual photoredox-nickel strategy for remote functionalization via iminyl radicals: radical ring-opening-arylation, -vinylation and -alkylation cascades
A divergent strategy for the remote arylation, vinylation and alkylation of nitriles is described.

Chem. Sci., 2019,10, 7728-7733
https://doi.org/10.1039/C9SC02616A
Diacetyl as a “traceless” visible light photosensitizer in metal-free cross-dehydrogenative coupling reactions
Minisci alkylation is of prime importance for its applicability in functionalizing diverse heteroarenes, which are core structures in many bioactive compounds.
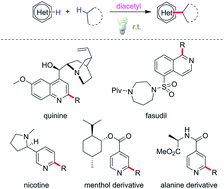
Chem. Sci., 2019,10, 5018-5024
https://doi.org/10.1039/C8SC05631E
Direct conversion of phenols into primary anilines with hydrazine catalyzed by palladium
A general and practical method to directly convert phenols into primary anilines with cheap and easy-to-handle hydrazine as the amine and hydride sources catalyzed by Pd/C.

Chem. Sci., 2019,10, 4775-4781
https://doi.org/10.1039/C9SC00595A
Desulfonative photoredox alkylation of N-heteroaryl sulfones – an acid-free approach for substituted heteroarene synthesis
Photoredox-mediated alkylation of heteroaryl sulfones under redox-neutral, acid-free conditions to create drug-like molecules.
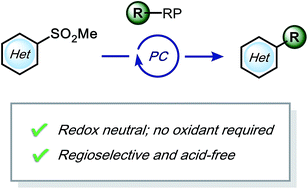
Chem. Sci., 2019,10, 4389-4393
https://doi.org/10.1039/C9SC00776H
BODIPY as electron withdrawing group for the activation of double bonds in asymmetric cycloaddition reactions
BODIPY as an EWG in asymmetric catalysis is presented.
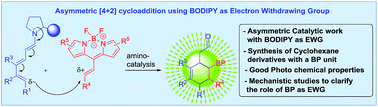
Chem. Sci., 2019,10, 4346-4351
https://doi.org/10.1039/C9SC00959K
Primary α-tertiary amine synthesis via α-C–H functionalization
A reactive ketimine intermediate was demonstrated to be intercepted by a variety of nucleophiles including organometallics and TMSCN.
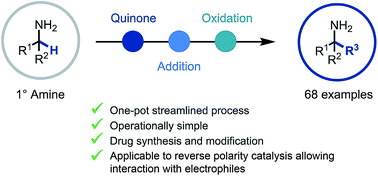
Chem. Sci., 2019,10, 3401-3407
https://doi.org/10.1039/C8SC05164J
Aromatic C–H amination in hexafluoroisopropanol
We report direct amination of electron-poor arenes and evaluate the crucial factors for the enhanced reactivity in hexafluoroisopropanol.

Chem. Sci., 2019,10, 2424-2428
https://doi.org/10.1039/C8SC04966A
Visible-light-mediated Minisci C–H alkylation of heteroarenes with unactivated alkyl halides using O2 as an oxidant
Herein, we report a protocol for direct visible-light-mediated Minisci C–H alkylation of heteroarenes with unactivated alkyl halides using molecular oxygen as an oxidant at room temperature.

Chem. Sci., 2019,10, 976-982
https://doi.org/10.1039/C8SC04892D
δ C–H (hetero)arylation via Cu-catalyzed radical relay
A radical relay strategy has been developed to enable selective δ C–H arylation. The approach employs a chiral copper catalyst, which serves the dual roles of generating an N-centered radical to promote intramolecular H-atom transfer, and then intercepting a distal C-centered radical for C–C bond formation with (hetero)aryl boronic acids.
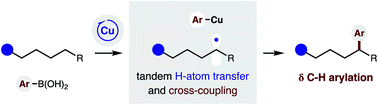
Chem. Sci., 2019,10, 1207-1211
https://doi.org/10.1039/C8SC04366C
Photoredox-mediated remote C(sp3)–H heteroarylation of free alcohols
We report an efficient and economical method for remote δ C(sp3)–H heteroarylation of free aliphatic alcohols using a hypervalent iodine PFBI-OH oxidant under photoredox catalysis.
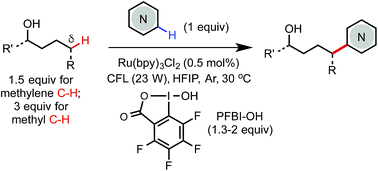
Chem. Sci., 2019,10, 688-693
https://doi.org/10.1039/C8SC04134B
Transition-metal free C–C bond cleavage/borylation of cycloketone oxime esters
An efficient transition-metal free C–C bond cleavage/borylation of cycloketone oxime esters has been described. In this reaction, the B2(OH)4 reagent not only served as the boron source but also acted as an electron donor source through formation of a complex with a DMAc-like Lewis base.

Chem. Sci., 2019,10, 161-166
https://doi.org/10.1039/C8SC03315C
Visible light-promoted ring-opening functionalization of unstrained cycloalkanols via inert C–C bond scission
Reported herein is a novel, useful, visible light-promoted ring-opening functionalization of unstrained cycloalkanols.
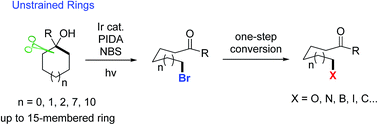
Chem. Sci., 2018,9, 5805-5809
https://doi.org/10.1039/C8SC01763H
Directed nickel-catalyzed 1,2-dialkylation of alkenyl carbonyl compounds
A substrate-directed approach to couple alkylzinc nucleophiles, alkyl halide electrophiles, and non-conjugated alkenes under nickel catalysis is described.

Chem. Sci., 2018,9, 5278-5283
https://doi.org/10.1039/C8SC01735B
Blue light-promoted photolysis of aryldiazoacetates
Aryldiazoacetates can undergo photolysis under blue light irradiation (460–490 nm) at room temperature and under air in the presence of numerous trapping agents, such as styrene, carboxylic acids, amines, alkanes and arenes, thus providing a straighforward and general platform for their mild functionalization.
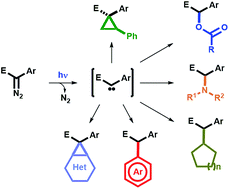
Chem. Sci., 2018,9, 5112-5118
https://doi.org/10.1039/C8SC01165F
Transition-metal-free decarboxylative bromination of aromatic carboxylic acids
Aromatic acids are converted into aryl bromides simply and efficiently via decarboxylation providing new depth and insight into Hunsdiecker-type reactivity.
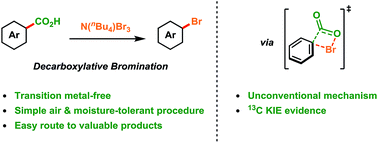
Chem. Sci., 2018,9, 3860-3865
https://doi.org/10.1039/C8SC01016A
Access to benzo-fused nine-membered heterocyclic alkenes with a trifluoromethyl carbinol moiety via a double decarboxylative formal ring-expansion process under palladium catalysis
Direct access to benzo-fused nine-membered heterocyclic alkenes with a trifluoromethyl carbinol moiety was achieved via a palladium-catalysis.
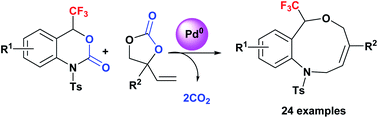
Chem. Sci., 2018,9, 3276-3281
https://doi.org/10.1039/C7SC05447E
A general method for the metal-free, regioselective, remote C–H halogenation of 8-substituted quinolines
An operationally simple, metal-free protocol for regioselective halogenation of a range of 8-substituted quinolines has been established using recyclable trihaloisocyanuric acids.
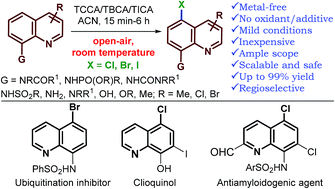
Chem. Sci., 2018,9, 1782-1788
https://doi.org/10.1039/C7SC04107A
Dual vicinal functionalisation of heterocycles via an interrupted Pummerer coupling/[3,3]-sigmatropic rearrangement cascade
An interrupted Pummerer coupling/[3,3]-sigmatropic rearrangement cascade allows the direct and metal free dual vicinal functionalisation of heterocycles. For example, C3 thio, C2 allyl indoles are prepared in one synthetic operation from the union of the parent indoles and allyl sulfoxides.
![Graphical abstract: Dual vicinal functionalisation of heterocycles via an interrupted Pummerer coupling/[3,3]-sigmatropic rearrangement cascade](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7SC04723A&imageInfo.ImageIdentifier.Year=2018)
Chem. Sci., 2018,9, 754-759
https://doi.org/10.1039/C7SC04723A
Nickel-catalyzed difunctionalization of allyl moieties using organoboronic acids and halides with divergent regioselectivities
We present herein a nickel-catalyzed dicarbofunctionalization of alkenes using readily available organoboronic acids and organic halides in a three-component fashion.
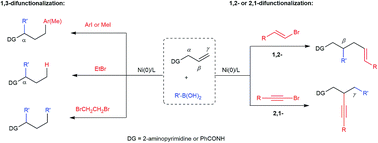
Chem. Sci., 2018,9, 600-607
https://doi.org/10.1039/C7SC03149A
About this collection
This specially curated collection pulls together some of the most popular articles from 2018 and 2019 in the field of organic chemistry. The collection presents some outstanding contributions to the field, ranging from dual vicinal functionalisation of heterocycles via an interrupted Pummerer coupling/[3,3]-sigmatropic rearrangement cascade to a review of C4-H indole functionalisation, and as with all Chemical Science articles – they are all completely free to access and read. We hope you enjoy browsing through this collection.
See also: