Themed collection 2014 Lab on a Chip Emerging Investigators

Emerging investigators: new challenges spawn new innovations
Guest editors Dino Di Carlo, Yanyi Huang and Helene Andersson-Svahn introduce this year's Emerging Investigators themed issue.

Lab Chip, 2014,14, 2599-2600
https://doi.org/10.1039/C4LC90058H
Contributors to Emerging Investigators 2014
Contributors to the Lab on a Chip Emerging Investigators 2014 themed issue.

Lab Chip, 2014,14, 2601-2604
https://doi.org/10.1039/C4LC90053G
Generating electric fields in PDMS microfluidic devices with salt water electrodes
Salt solution electrodes provide a simpler and equally functional alternative to metal electrodes for applying electric fields in PDMS.
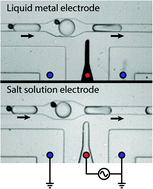
Lab Chip, 2014,14, 2605-2609
https://doi.org/10.1039/C4LC00078A
A microchip platform for interrogating tumor–macrophage paracrine signaling at the single-cell level
An antibody barcode microchamber array chip permits the measurement of secreted proteins from pairs of individual tumor and macrophage cells and quantifying paracrine signaling-induced functional changes.
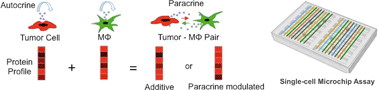
Lab Chip, 2014,14, 3582-3588
https://doi.org/10.1039/C4LC00676C
AC electric field induced dipole-based on-chip 3D cell rotation
First report on 3D rotation of cells using alternating current electric field on a single, open-top, and cost effective biochip.
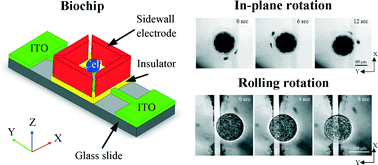
Lab Chip, 2014,14, 2717-2727
https://doi.org/10.1039/C4LC00312H
Engineering interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as the sacrificial template
We engineer interconnected 3D vascular networks in hydrogels using molded sodium alginate lattice as sacrificial templates. The size and morphology of simulated vascular networks were well controlled and a fully-developed endothelial layer was formed.
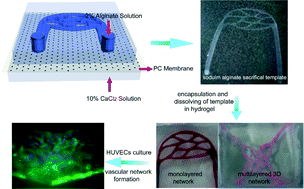
Lab Chip, 2014,14, 2709-2716
https://doi.org/10.1039/C4LC00069B
Dynamics of counterion-induced attraction between vimentin filaments followed in microfluidic drops
The dynamics of intermediate filament network formation are studied in microfluidic drops at high temporal and spatial resolution.
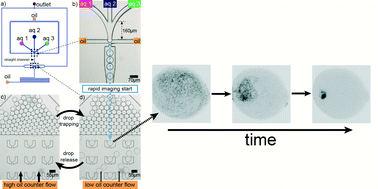
Lab Chip, 2014,14, 2681-2687
https://doi.org/10.1039/C3LC51418H
Automated single cell microbioreactor for monitoring intracellular dynamics and cell growth in free solution
The single cell microbioreactor allows for precise and rapid control over the growth environment for cells cultured in free solution, thereby facilitating direct analysis of intracellular dynamics.
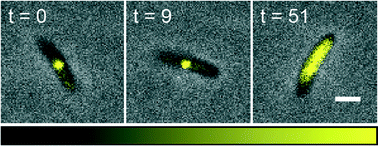
Lab Chip, 2014,14, 2688-2697
https://doi.org/10.1039/C4LC00057A
Liquid polystyrene: a room-temperature photocurable soft lithography compatible pour-and-cure-type polystyrene
In this paper we introduce “liquid polystyrene,” a castable photocurable polystyrene prepolymer, for microfluidic prototyping. Using this material, polystyrene, an important polymer in cell biology will become accessible via soft lithography replication.
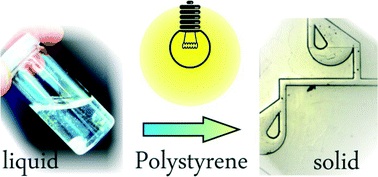
Lab Chip, 2014,14, 2698-2708
https://doi.org/10.1039/C4LC00045E
A centrifugal fluidic immunoassay for ocular diagnostics with an enzymatically hydrolyzed fluorogenic substrate
The novelty of this platform is the combination of sedimentation principles with an enzymatically hydrolyzed fluorogenic substrate to develop a rapid immunoassay.
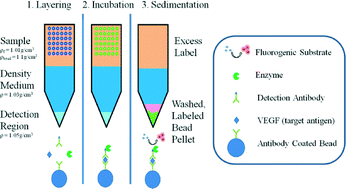
Lab Chip, 2014,14, 2673-2680
https://doi.org/10.1039/C4LC00279B
A simple strategy for in situ fabrication of a smart hydrogel microvalve within microchannels for thermostatic control
A smart hydrogel microvalve is in situ fabricated within a microchip and integrated in the flow circulation loop of a micro-heat-exchanging system for thermostatic control.
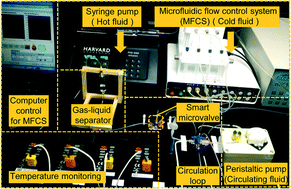
Lab Chip, 2014,14, 2626-2634
https://doi.org/10.1039/C4LC00039K
Micropatterned biofilm formations by laminar flow-templating
We present a flow-templating micro-bioreactor as a new concept for controlled patterning of linear biofilm formations. Experiments and simulations comprehensively exploit control parameters to grow biofilm patterns with controllable dimensions. The paper concludes with a study of biofilm growth rates under well-defined shear stress environments.
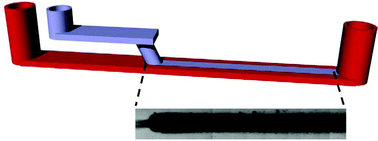
Lab Chip, 2014,14, 2666-2672
https://doi.org/10.1039/C4LC00084F
DNA-functionalized hydrogels for confined membrane-free in vitro transcription/translation
A microfluidically fabricated DNA-functionalized porous hydrogel based on hyaluronic acid is applied to spatially confine in vitro gene expression in a membrane-free compartment that facilitates precise control over protein production rates and is envisioned to serve as a basis to design protocells.

Lab Chip, 2014,14, 2651-2656
https://doi.org/10.1039/C3LC51427G
Clog-free cell filtration using resettable cell traps
A microfluidic cell separation mechanism created using constrictions with adjustable size that can selectively capture and release cells, thereby enabling high throughput size and deformability based cell separation without clogging.
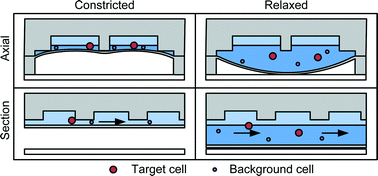
Lab Chip, 2014,14, 2657-2665
https://doi.org/10.1039/C4LC00306C
Magnetically controllable 3D microtissues based on magnetic microcryogels
Microtissues on the scale of several hundred microns are a promising cell culture configuration resembling the functional tissue units in vivo.
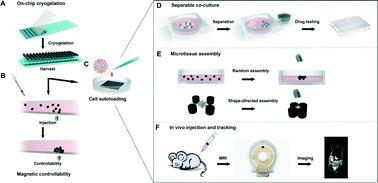
Lab Chip, 2014,14, 2614-2625
https://doi.org/10.1039/C4LC00081A
Make it spin: individual trapping of sperm for analysis and recovery using micro-contact printing
Trapping of single sperm cells on protein islands for subsequent motility analysis as an aid to choose the best one.
Lab Chip, 2014,14, 2635-2641
https://doi.org/10.1039/C4LC00050A
A 1024-sample serum analyzer chip for cancer diagnostics
A microarray/microfluidic platform measures four protein biomarkers in 1024 blood serum samples for 4096 assays per device with a limit-of-detection of ~1 pM.
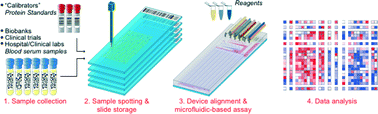
Lab Chip, 2014,14, 2642-2650
https://doi.org/10.1039/C3LC51153G
Print your own membrane: direct rapid prototyping of polydimethylsiloxane
We present a technique to 3D print unprecedented microfluidic PDMS device geometries, circumventing the need for photolithography masks and sacrificial molds.

Lab Chip, 2014,14, 2610-2613
https://doi.org/10.1039/C4LC00320A
About this collection
Lab on a Chip is committed to supporting early career scientists, and it is because of this that we are both proud and very pleased to introduce the 2014 edition of our emerging investigators issue, which celebrates the best and brightest amongst early career miniaturisation scientists around the world. This issue was guest edited by Dino di Carlo, Helene Andersson-Svahn and Yanyi Huang who worked hard to create this issue and ensure that its content was of the highest quality. Please read the articles below to see how our emerging investigators are contributing to the world of miniaturisation.