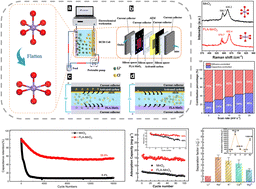
In this study, the incorporation of Mg2+ induced the transformation of MnO2 from the original cubic [MnO6] octahedron to a flattened conformation (FLA-MnO2). The flattened conformation mitigated structural deformation of the [Mn3+·O6] octahedron by inhibiting the J–T aberration during the reduction process of Mn4+, thereby improving the selective adsorption capacity of lithium. When used in hybrid capacitive deionization (HCDI), FLA-MnO2 exhibited a high Li+ adsorption capacity of 30.14 mg g−1 in 32.74 mg L−1 Li+ ion solution, with low energy consumption of 0.45 Wh g−1. Notably, the FLA-MnO2‖AC HCDI cell exhibited high stability, maintaining 82% of its capacity over 100 cycles. This was evidenced by the 82% capacity retention and low Mn loss (1.3%) over 100 cycles. Finally, the excellent selective extraction performance of Li+ ions was demonstrated in Lop Nor, the low-grade original brine of the XieLi salt flats. The calculated separation factors for Li+/Na+, Li+/K+, Li+/Ca2+, and Li+/Mg2+ reached impressive values of 4.37, 3.75, 3.24, and 2.11, respectively, making the Lop Nor a candidate electrode for lithium extraction from low-grade raw brines.