Nickel/photoredox dual-catalyzed reductive cross-coupling of aryl halides and aldehydes†
Abstract
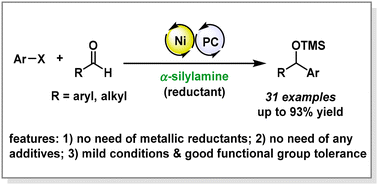
A straightforward reductive cross-coupling of aryl halides and aldehydes to afford silyl-protected secondary alcohols is reported. The catalytic system features the use of nickel/photoredox dual catalysis and α-silylamine as a mild, easily accessible organic reductant, thus enabling the coupling process to occur smoothly under mild conditions without the necessity of pre-preparing highly reactive organometallic reagents and using stoichiometric amounts of metal-based reductants (such as Mn and Zn). The key to the success of this reductive coupling is to identify α-silylamine as a bifunctional organic reductant that simultaneously serves as a reducing agent and a transmetallic reagent to restore the nickel catalyst. The reaction shows a broad substrate scope and tolerates various functional groups, and a variety of (hetero)aryl and aliphatic aldehydes are compatible, thus providing a complementary approach towards Zn/Mn-mediated reductive coupling systems.
- This article is part of the themed collection: Organic Chemistry Frontiers Emerging Investigator Series 2024–2025


 Please wait while we load your content...
Please wait while we load your content...