Photochemical mechanistic study of hexafluorobenzene involving the low-lying states†
Abstract
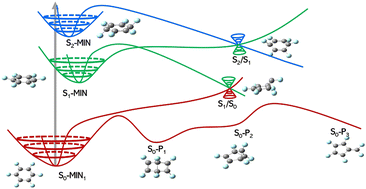
The photochemical isomerization and nonradiative decay processes of hexafluorobenzene (HFB) were investigated theoretically to gain insights into its photochemical mechanism and the perfluoro effect. A complete mechanistic scheme is presented through the characterization of all the possible minima and transition states of the S0, S1, and S2 states at the CASPT2/6-311G**//CAS(6,7)/6-31G* level. On the S0 potential energy surface, HFB could isomerize to three different products [Dewar-HFB (S0-P1), benzvalene-HFB (S0-P2), and fulvene-HFB (S0-P3)]. Following excitation to the S2 state with the perpendicular π → σ* transition, a chair-type minimum with Cs symmetry was found on the S2 potential energy surface. The adjacent S2/S1 conical intersection was immediately accessible from the S2 minimum. The nature of the S1 state was confirmed to have a π → π* character. Both the S2 and S1 photochemistries of HFB yielded Dewar-HFB via the S1/S0 conical intersection. The regeneration of the S0 state from the S1 and T2 states via intersystem crossing or internal conversion was also revealed.


 Please wait while we load your content...
Please wait while we load your content...