Construction of thioglycoside bonds via an asymmetric organocatalyzed sulfa-Michael/aldol reaction: access to 4′-thionucleosides†
Abstract
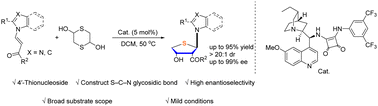
Thioglycoside bond formation via an asymmetric sulfa-Michael/aldol reaction of (E)-β-nucleobase acrylketones and 1,4-dithiane-2,5-diol has been achieved with a cinchona alkaloid-derived bifunctional squaramide chiral catalyst. Diverse purine, benzimidazole, and imidazole substrates are well tolerated and generate 4′-thionucleoside derivatives containing three contiguous stereogenic centers with excellent results (30 examples, up to 97% yield, >20 : 1 dr and up to 99% ee). Moreover, the novel strategy provides an efficient and convenient synthetic route to construct chiral 4′-thionucleosides.
- This article is part of the themed collection: Chemical Communications HOT Articles 2024


 Please wait while we load your content...
Please wait while we load your content...