Abstract
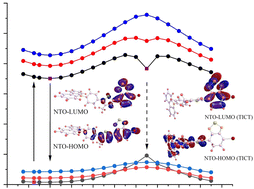
We performed a combined experimental and ab initio study of the excited state dynamics of a carbazole–bromobenzothiadiazole (CBB) fluorophore, a molecule that was designed to exhibit strong solvatochromic shifts due to large charge separation in the minimum of the excited state potential energy surface. While the experimental Stokes shifts – obtained here through Excitation Emission Matrix spectroscopy – showed the expected large solvatochromic shift, we found no evidence that this shift is induced by a twisted-internal charge transfer (TICT) state as had been previously (and reasonably) predicted. Instead, ab initio calculations using TD-DFT and the CAM-B3LYP/6-31G+(d,p) (IEF-PCM) model explained the shift semi-quantitatively using a moderate charge separation in the excited state combined with small contributions of solvent-induced dipole moments in CBB that depend on the solvent polarity. While a TICT state could be identified as a local minimum on the S1 potential energy surface through its large dipole along the donor–acceptor axis and by examination of the natural transition orbitals, the global minimum on the S1 surface is close to the Franck–Condon region of excitation and much more accessible. This study highlights some of the complexities in identifying TICT states from experimental observations and frontier orbitals, alone.


 Please wait while we load your content...
Please wait while we load your content...