Synthesis, optical and redox attributes of core-/bay-substituted thionated NDIs, PDIs and their diverse radical anions†
Abstract
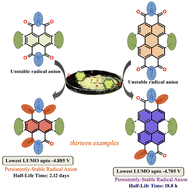
Organic radical anions due to their wide-ranging applications can offer an exciting platform to investigate their ease of generation, structure–stability correlation, color modulation, etc. Radical anions of core-/bay-substituted thionated arylene diimides have not been studied to date. These can offer highly divergent structures, present opportunities to greatly tune their lifetimes and result in emergent colors due to their tunable doublet–doublet transitions. Herein, we synthesized four classes of NDI-/PDI-based molecular systems (classes I–IV), which have one/two/three S atoms at the imide positions. In class I, thionated NDIs without core groups are considered. In classes II and III, thionated NDIs are substituted at the core positions with halogen atoms or cyano groups or chalcogen atoms. In class IV, we considered mono-/bis-thionated PDIs with halogen atoms at the 2,7-bay-positions. Interestingly, we isolated two pairs of thionated PDI regioisomers having subtle differences in their redox and opto-electronic properties. The radical anions formed from these classes of molecules are diversely colored, e.g., brown, purple, violet, blue and green. The half-life of these radical anions could be significantly varied based on the degree of thionation and the core-/bay-substitutions and can offer promise in redox-catalysis, as NIR absorbers, organic reducing agents, etc.
- This article is part of the themed collection: 2023 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...