Regioselective acylation attaching aromatic substituents in simple nonfullerene acceptors for efficient organic solar cells†
Abstract
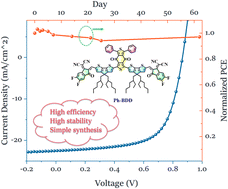
Aromatic substituent engineering of side chains plays a multi-functional role in molecular structure and properties. In this work, the key aromatic substituted cores of simple nonfullerene acceptors are realized through efficient regioselective acylation. More specifically, two simple nonfullerene acceptors Ph-BDD and C4Ph-BDD with different phenyl-substituted benzodithiophenedione as the core moiety were synthesized. Ph-BDD with bare benzene rings exhibits stronger aggregation and crystallinity than C4Ph-BDD with butyl benzene rings. Moreover, the single crystal structure of Ph-BDD reveals that a three-dimensional interpenetrating network can be formed due to the compact π–π stacking between the adjacent end-capping groups. With polymer PM6 as the donor, Ph-BDD exhibits a much higher power conversion efficiency (PCE) of 13.64%, attributed to the more balanced carrier mobilities and better phase separation in blend morphology, compared to that of C4Ph-BDD (PCE of 11.37%). Besides, both Ph-BDD- and C4Ph-BDD-based devices can retain 97% of their original efficiency after being stored in air for more than 60 days. Overall, feasible regioselective acylation is of significant importance to flourish of BDD with various aromatic substituents, which has great potential in stable and high-performing nonfullerene solar cells.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...