Highly efficient and scalable total syntheses and stereochemical assignment of potent anti-inflammatory pesimquinolones I and J†
Abstract
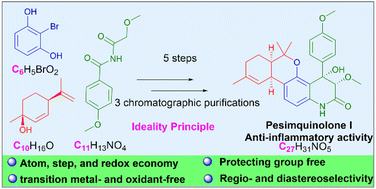
Based on the goals of sustainability and environmentally conscious science, great progress has been made in the field of green chemical synthesis, but a synthesis that combines high levels of step economy with high levels of efficiency and scalability has remained elusive. In this research, highly efficient and scalable total synthesis of potent anti-inflammatory pesimquinolone I (1) with a unique 6/6/6/6 tetracyclic core was accomplished starting from 2-bromoresorcinol and (1S,4R)-menthadienol in 5 steps. The approach adheres to the ideality principle, and the concepts of atom, step and redox economy, green chemistry, selectivity, and protecting group-free syntheses have been introduced. The synthetic strategy relies on a Lewis acid catalyzed Friedel–Crafts alkylation to form the tricyclic intermediate, a regioselective insertion of arynes into unsymmetric imides and a diastereoselective aldol cyclization to construct the tetracyclic skeleton. Syntheses and stereochemical assignment of the stereoisomer of pesimquinolone I and its analogues (15–19) were also accomplished. Compound 18 showed potent NO inhibitory effects with an IC50 value of 0.44 μM, which was stronger than that of the positive control (indomethacin, IC50 = 50.00 μM), and thus represents a potentially promising lead for anti-inflammatory drug discovery.


 Please wait while we load your content...
Please wait while we load your content...