Versatile access to nitrogen-rich π-extended indolocarbazoles via a Pictet–Spengler approach†
Abstract
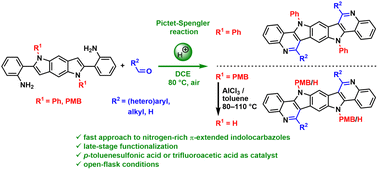
The Pictet–Spengler reaction was applied to synthesize benzobispyrrolo[3,2-c]quinolines as new scaffolds for organic electronics. The addressed compounds are nitrogen-enriched analogues of π-extended indolocarbazoles formally accessed by the isosteric replacement of CH moieties by nitrogen atoms. The straightforward and robust methodology allows easy access to these heptacycles with the possibility of late-stage functionalization. 17 new compounds were synthesized, fully characterized and their properties were investigated by X-ray crystallography, photophysical measurements and computational methods. The study evaluated the effect of different substituents on the observed photophysical and electronical properties. Comparison with π-extended indolocarbazoles showed that the introduction of two nitrogens to the molecule core results in a significant decrease of the HOMO and LUMO energies and an increase of the optical and HOMO–LUMO gaps.
- This article is part of the themed collection: 2023 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...