Thia-Michael addition: the route to promising opportunities for fast and cysteine-specific modification
Abstract
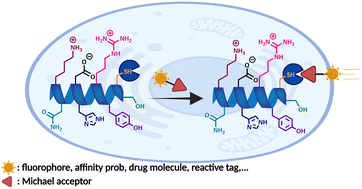
Over the last few decades, design and discovery of chemical reactions that enable modification of proteins at pre-determined sites have been the focus of synthetic organic chemists. As an invaluable tool, the site-and chemoselective functionalization of peptides and proteins offers an exciting opportunity for creating high-value multicomponent conjugates with diverse applications in life sciences and pharmacology. In recent years, multiple strategies have emerged that target natural amino acids directly or convert them into other reactive species for further ligations. However, reactivity and selectivity are still key issues in the current state of chemical modification methodologies. Cysteine is one of the least abundant amino acids and exhibits unique chemistry of the thiol or thiolate group which makes it susceptible to a series of post-translational modifications. The thia-Michael “click” addition reactions, which can proceed under facile conditions provide a promising way for thiol-selective modification of cysteine-containing proteins. In this review, we summarize various reactions for cysteine-selective peptide and protein modification, focus on thia-Michael “click” addition reactions, elaborate on their historical perspective and mechanism, and highlight their applications in modifying biomolecules in a site-specific way.
- This article is part of the themed collection: Celebrating the 20th anniversary of Organic & Biomolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...