Directing oxygen reduction reaction selectivity towards hydrogen peroxide via electric double layer engineering†
Abstract
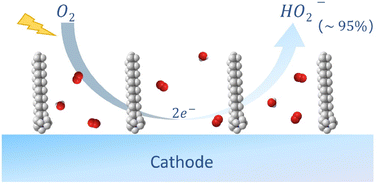
The electrochemical oxygen reduction reaction (ORR) has been recognized as a promising alternative for the sustainable production of H2O2. Here, we report a facile and effective strategy to promote ORR selectivity towards the 2e− product H2O2via electric double layer engineering. Specifically, in a model system using immobilized cobalt phthalocyanine as the electrocatalyst, H2O2 selectivity has been improved from below 60% to over 93%, and the intrinsic activity for H2O2 formation has been enhanced by more than 3 times upon the introduction of a cationic surfactant (i.e., cetyltrimethylammonium bromide, CTAB) into the electrolyte. Based on detailed kinetics analysis, we conclude that the accelerated H2O2 formation rate results from the reduced charge transfer resistance in the rate limiting step and the promoted oxygen uptake rate. We propose that the electric field strength across the electric double layer is enhanced via the self-assembled single-tail cationic surfactant layer at the electrode/electrolyte interface, which is the origin of the enhancement of the 2e− ORR performance.
- This article is part of the themed collection: Nanoscale 2023 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...