An experimental and computational study of a low-temperature electrolyte design utilizing iodide-based ionic liquid and butyronitrile
Abstract
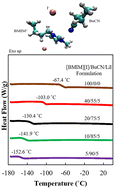
Wide liquidus range electrolytes are in popular demand to extend the operating temperature windows of electrochemical devices. Different from conventional electrolytes, which dissolve salt in molecular liquids, highly tunable ionic liquids (ILs) offer attractive properties as competitive candidates in recent electrolyte developments. While IL-based electrolytes showcase satisfying performances at high and room temperatures, their functionality at low temperatures remains a challenge with the most reported progress around −40 to −70 °C. Herein, we present experimental and computational studies on low-temperature liquid electrolyte systems consisting of iodide containing IL, 1-butyl-3-methylimidazolium iodide ([BMIM][I]), nitrile solvents, acetonitrile (ACN) and butyronitrile (BuCN), and lithium iodide (LiI). While the [BMIM][I]/ACN/LiI system exhibits first-order phase transitions with increasing ACN concentration, [BMIM][I]/BuCN/LiI systems remain as glass-forming liquids over a wide composition range. More impressively, [BMIM][I]/BuCN/LiI-5/90/5 possesses an extremely low glass transition temperature of −152 °C, which marks the lowest liquidus range limit of reported IL-based mixtures to the best of our knowledge. Correspondingly, enhancements in transport properties are observed, especially at temperatures below −40 °C in comparison to our previous work. Moreover, iodide/triiodide electrochemistry in BuCN is validated as active and stable within the identified potential window. We attribute the bulk property evolution of the designed electrolytes to microscopic intermolecular interaction optimization, specifically, solvation of BuCN around the imidazolium cation via hydrogen bonding, as characterized using Fourier transform infrared (FTIR) and Raman spectroscopies. Furthermore, the effects of BuCN on the ion and molecular ordering are investigated through molecular dynamics simulation, which elucidates a different mechanism from IL/water mixtures. This work presents IL/BuCN systems with an exceptionally wide liquidus range and improvements in transport, which not only benefits iodide/triiodide-based technologies but also serves as a foundation for further task-specific optimization to support electrochemical devices at ultralow temperatures.


 Please wait while we load your content...
Please wait while we load your content...