A green recycling process designed for LiFePO4 cathode materials for Li-ion batteries†
Abstract
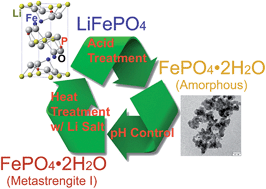
A green process route to recycle LiFePO4/C electrode materials is proposed in this work. First, a robust strategy to synthesize LiFePO4/C cathode materials from a precursor of a crystalline FePO4·2H2O phase (metastrengite I) is presented. In order to prepare crystalline FePO4·2H2O, a solution precipitation route is adapted, where the reaction conditions such as temperature and pH are precisely controlled. Among various heat treatment temperatures to calcine our prepared FePO4·2H2O with lithium sources, we find that LiFePO4/C cathode materials synthesized at 700 °C deliver a maximum discharge capacity of 168.51 mA h g−1 at 0.1 C (1 C rate = 170 mA h g−1) with a capacity retention of 99.36% after the 25th cycle at 1 C. Furthermore, commercially available LiFePO4 powders and recovered LiFePO4 electrode materials from spent batteries are both tested with our developed recycling process, where we decompose LiFePO4 powders/electrodes to prepare crystalline FePO4·2H2O, and then re-synthesize LiFePO4/C cathode materials. In both cases, our recycled LiFePO4/C exhibits a very comparable discharge capacity of ∼140 mA h g−1 at 1 C with a capacity retention of ∼99%.

 Please wait while we load your content...
Please wait while we load your content...