Visual assay of Escherichia coli O157:H7 based on an isothermal strand displacement and hybrid chain reaction amplification strategy†
Abstract
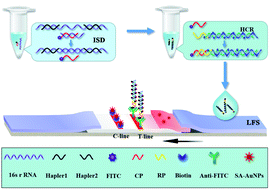
Here, we describe a simple, sensitive, and enzyme-free method for visual point-of-care detection of 16S rRNA of Escherichia coli O157:H7 based on an isothermal strand displacement-hybrid chain reaction (ISD-HCR) and lateral flow strip (LFS). In this study, the secondary structure of 16S rRNA of E. coli O157:H7 was unwound by two helper oligonucleotides to expose the single-strand-specific nucleic acid sequence. The free specific sequence promoted the toehold-mediated strand displacement reaction to output a large number of FITC-labeled single-stranded DNA probes (capture probe [CP]). The 3′-end sequence of the reporter probe propagated a chain reaction of hybridization events between the two hairpin probes modified with biotin to form long nicked DNA polymers with multiple biotins (RP-HCR complexes); the free CP and RP-HCR complexes then form CP/RP-HCR complexes. The biotin-labeled double-stranded DNA CP/RP-HCR polymers then introduced numerous streptavidin (SA)-labeled gold nanoparticles (AuNPs) on the LFS. The accumulation of AuNPs produced a characteristic red band, which enabled visual detection of changes in the signal of 16S rRNA of E. coli O157:H7. The current approach could detect E. coli O157:H7 at concentrations as low as 102 CFU mL−1 without instrumentation. This approach thus provides a simple, sensitive, and low-cost tool for point-of-care detection of pathogenic bacteria, especially in resource-limited countries.


 Please wait while we load your content...
Please wait while we load your content...