Organophotocatalytic silyl transfer of silylboranes enabled by methanol association: a versatile strategy for C–Si bond construction†
Abstract
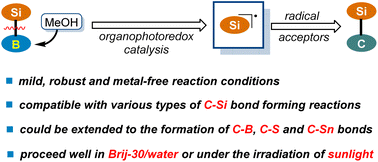
Development of a mild, robust and metal-free catalytic approach for silyl transfer of silylboranes is critical for the advancement of modern organosilicon chemistry given their powerful capacity in the construction of C–Si bonds. Herein, we wish to disclose a visible light-induced organophotocatalytic strategy, where methanol association with boron atoms enables the photocatalytic generation of silyl radicals. Notably, the protocol is capable of accommodating a wide range of radial acceptors through slightly tuning the reaction conditions, which allows the formation of various types of C–Si bonds. The preparative power of the transformations has been further highlighted in a number of complex settings, including late-stage functionalization and radical cascade reactions. Furthermore, this technology could be extended to the construction of C–B, C–S and C–Sn bonds, thus offering a versatile platform for bond activation and connection of main group elements. The green aspect of the reaction has also been demonstrated by using Brij-30/water as a reaction solvent or sunlight as an alternative energy source.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...