An insight into metal organic framework derived N-doped graphene for the oxidative degradation of persistent contaminants: formation mechanism and generation of singlet oxygen from peroxymonosulfate†
Abstract
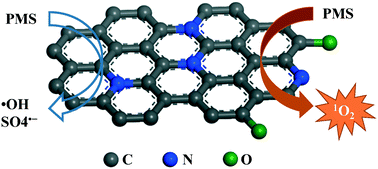
The synthesis of carbonaceous materials from a metal organic framework (MIL-100), organic linker and N-precursor was comprehensively investigated, and the structures of the products were characterized. It was found that simple pyrolysis of mixed MIL-100 (Fe)/dicyandiamide (DCDA) could produce nitrogen-doped graphene (N-graphene). The N-graphene showed excellent performances in peroxymonosulfate (PMS) activation, which were superior to those of counterparts of graphene, iron(II, III) oxide, manganese(IV) oxide and cobalt(II, III) oxide. With PMS activation, N-graphene exhibited efficient catalytic degradation of various organic pollutants such as phenol, 2,4,6-trichlorophenol (TCP), sulfachloropyridazine (SCP) and p-hydroxybenzoic acid (PHBA). Electron paramagnetic resonance (EPR) spectroscopy and radical quenching tests were employed to investigate the PMS activation and organic degradation processes. It was found that singlet oxygen (1O2) was mainly produced during the activation of PMS by N-graphene, and contributed to the catalytic oxidation instead of sulfate and/or hydroxyl radicals. These findings provide new insights into PMS activation by metal-free carbon catalysis.
- This article is part of the themed collection: Environmental Science: Nano 2017 Most Downloaded Articles


 Please wait while we load your content...
Please wait while we load your content...