Graphene quantum dots as a highly efficient electrocatalyst for lithium–oxygen batteries†
Abstract
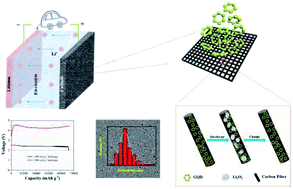
Emerging graphene quantum dots (GQDs) materials have attracted worldwide attention in biological, optoelectronic and energy-related applications because of their unique features. Herein, we successfully synthesized glucose-derived GQDs through a hydrothermal process, which were further employed as an efficient cathodic catalyst in non-aqueous lithium–oxygen batteries for the first time. The GQDs possessed an average size of about 3 nm (less than 10 layers), and exhibited excellent water/ethanol solubility, which was beneficial for the impregnation process. The distinct G band in the Raman spectrum of the GQDs demonstrated their crystalline core, and their unique optical properties suggested the existence of a self-passivated layer outside their core. Furthermore, a GQDs-impregnated cathode was fabricated, which delivered an ultrahigh capacity of 68 900 mA h g−1 under a current density of 1400 mA g−1 in a 1 M LiTFSI/TEGDME electrolyte system. Moreover, the GQDs-impregnated cathode showed a quite good stability under a current density of 2000 mA g−1. Under a limited capacity of 1000 mA h g−1, it could cycle for 300 cycles without obvious decay. These results strongly suggest that the GQDs materials have good application prospect in lithium–oxygen battery systems as a superior cathodic catalyst.


 Please wait while we load your content...
Please wait while we load your content...