The effect of ginger (Zingiber officinale) supplementation on clinical, biochemical, and anthropometric parameters in patients with multiple sclerosis: a double-blind randomized controlled trial†
Abstract
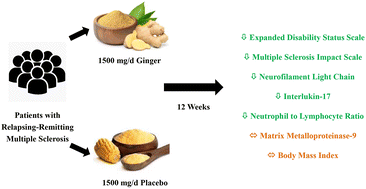
Introduction: different lines of evidence have shown that ginger administration may be beneficial for patients with multiple sclerosis (MS). Therefore, we aimed to investigate the effect of ginger supplementation on disability, physical and psychological quality of life (QoL), body mass index (BMI), neurofilament light chain (NfL), interlukin-17 (IL-17), matrix metalloproteinase-9 (MMP-9), and neutrophil to lymphocyte ratio (NLR) in patients with relapsing-remitting MS. Methods: this was a 12 week double-blind parallel randomized placebo-controlled trial with a 3 week run-in period. The treatment (n = 26) and control (n = 26) groups received 500 mg ginger and placebo (corn) supplements 3 times daily, respectively. Disability was evaluated using the Expanded Disability Status Scale (EDSS). QoL was rated using the Multiple Sclerosis Impact Scale (MSIS-29). BMI was calculated by dividing weight by height squared. Serum levels of NfL, IL-17, and MMP-9 were measured using the enzyme-linked immunosorbent assay. NLR was determined using a Sysmex XP-300™ automated hematology analyzer. All outcomes were assessed before and after the intervention and analyzed using the intention-to-treat principle. Results: in comparison with placebo, ginger supplementation caused a significant reduction in the EDSS (−0.54 ± 0.58 vs. 0.08 ± 0.23, P < 0.001), the MSIS-29 physical scale (−8.15 ± 15.75 vs. 4.23 ± 8.46, P = 0.001), the MSIS-29 psychological scale (−15.71 ± 19.59 vs. 6.68 ± 10.41, P < 0.001), NfL (−0.14 ± 0.97 vs. 0.38 ± 1.06 ng mL−1, P = 0.049), IL-17 (−3.34 ± 4.06 vs. 1.77 ± 6.51 ng L−1, P = 0.003), and NLR (−0.09 ± 0.53 vs. 0.53 ± 1.90, P = 0.038). Nevertheless, the differences in BMI and MMP-9 were not significant between the groups. Conclusion: ginger supplementation may be an effective adjuvant therapy for patients with relapsing-remitting MS.


 Please wait while we load your content...
Please wait while we load your content...