Melting properties of AgxPt1−x nanoparticles
Abstract
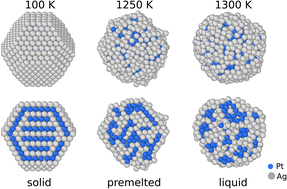
At the nanoscale, materials exhibit unique properties that differ greatly from those of the bulk state. In the case of AgxPt1−x nanoalloys, we aimed to study the solid–liquid transition of nanoparticles of different sizes and compositions. This system is particularly interesting since Pt has a high melting point (2041 K compared to 1035 K for Ag) which could keep the nanoparticle solid during different catalytic reactions at relatively high temperatures, such as we need in the growth of nanotubes. We performed atomic scale simulations using a semi-empirical potential implemented in a Monte Carlo code at constant temperature and chemical composition in a canonical ensemble. We observed that the melting temperature decreases with decreasing size (pure systems and alloys) and increasing Ag content. We show that the melting systematically passes through an intermediate stage with a crystalline core (pure platinum or mixed PtAg depending on the composition) and a pure silver liquid skin, which strongly questions the idea of having a faceted solid particle in catalytic reactions for carbon nanotube synthesis.
- This article is part of the themed collection: Nanoalloys: recent developments and future perspectives


 Please wait while we load your content...
Please wait while we load your content...