Facet-specific cation exchange and heterogeneous transformation of cadmium sulfide nanoparticles induced by Cu(ii)†
Abstract
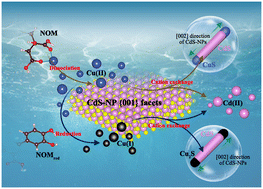
Cadmium sulfide nanoparticles (CdS-NPs) can be formed prevalently in the environment via microbial transformation of anthropogenic Cd(II). The behavior of CdS-NPs in various natural processes is a key factor controlling their stability and the release of toxic Cd(II). However, the microscopic kinetics are poorly understood due to the complexity of the intrinsic morphology- and facet-dependent NP surface processes, which are further complicated by environmental matrix effects. In this study, we investigated the stability of three CdS-NPs (CdS-sphere, CdS-rod, and CdS-sheet) and one nanosized biogenic CdS (Bio-CdS NPs) in Cu(II) spiked solutions using combined kinetic, spectroscopic, HRTEM and DFT approaches. Cd(II) was quickly released (2.578–14.547 mmol L−1 h−1 m−2) by Cd/Cu cation exchange, especially for CdS-sheet (5.945–13.789 mmol L−1 h−1 m−2), and the morphologies of CdS-NPs were preserved after the cation exchange. Additionally, the cation exchange on CdS-NP surfaces was influenced by the coexisting DOM and reducing substances. In terms of DOM, the complexation of Cu(II) with DOM decreased the free Cu(II) ions in solutions, thus the cation exchange on the CdS-NP surfaces with Cu(II) was inhibited in the presence of DOM. Conversely, the presence of reducing substances promoted the dissolution of CdS-NPs due to the formation of chalcophile Cu(I), forming more chemically stable Cu2S and releasing the lattice Cd(II). The results of HRTEM and DFT calculations confirmed that the cation exchange occurred preferentially on the CdS-NP {001} facet, forming CdS/CuS heterostructures with covellite CuS growing at the ends along the [002] direction of CdS. Altogether, these findings have important geochemical significance as the facet-dependent transformation of metal sulfides is consequential in complex natural and anthropogenic environments.
- This article is part of the themed collection: Environmental Science: Nano Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...