Flavonol dioxygenation catalysed by cobalt(ii) complexes supported with 3N(COO) and 4N donor ligands: a comparative study to assess the carboxylate effects on quercetin 2,4-dioxygenase-like reactivity†
Abstract
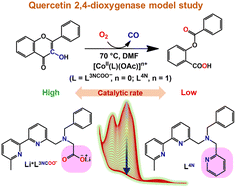
Two new cobalt(II)-acetato complexes, [CoII(L3NCOO)(OAc)]·0.5H2O (1OAc·0.5H2O) and [CoII(L4N)(OAc)](PF6) (2OAc(PF6)), were synthesised using ligands L3NCOO− (Li+L3NCOO− = lithium 2-(benzyl((6′-methyl-[2,2′-bipyridin]-6-yl)methyl)amino)acetate) and L4N (N-benzyl-1-(6′-methyl-[2,2′-bipyridin]-6-yl)-N-(pyridin-2-ylmethyl)methanamine), respectively, to mimic the functional activity of cobalt(II)-quercetin-2,4-dioxygenase (CoII-2,4-QD). Additionally, Co(II)-flavonolato ternary complexes, [CoII(L3NCOO)(fla)]·H2O (1fla·H2O) and [CoII(L4N)(fla)](PF6) (2fla(PF6)), were synthesised as enzyme–substrate models. All four complexes were thoroughly characterised by elemental analyses and spectroscopic methods. Structural characterisation was performed for 1OAc·0.5H2O, 2OAc(PF6)·CH2Cl2 and 2fla+ with a perchlorate counter anion, 2fla(ClO4)·1.5H2O. Furthermore, density functional theory (DFT) calculations, time-dependent DFT (TD-DFT) and molecular orbital (MO) analysis were performed for the flavonolato adducts 1fla and 2fla+. The catalytic activities of complexes 1OAc·0.5H2O and 2OAc(PF6) in the oxygenative degradation of flavonol (multiple-turnover reactions) were investigated at 70 °C in DMF to determine the effect of the carboxylate substituent over a pyridyl donor residue on reactivity. Complex 1OAc·0.5H2O showed a higher catalytic rate than complex 2OAc(PF6). The same reactivity order was observed for single-turnover dioxygenation reactions with ternary complexes (1fla > 2fla+). The formation constants (Kf) of 1fla and 2fla+ species are comparable, implying that catalyst–substrate adduct formation occurs in similar amounts for both catalytic reactions. Therefore, the Kf values have a similar impact on reactivities. However, the oxidation potential of the bound fla−/fla˙ couple in 1fla is considerably lower than that in 2fla+. DFT calculations predicted that the negatively charged carboxylate group of ligand L3NCOO− determines the higher reactivity of 1fla with dioxygen by decreasing the oxidation potential of the bound fla−/fla˙ couple. During the dioxygenation process, the reactive Co(II)-bound flavonoxy radical was generated via single-electron transfer from the coordinated fla− to dioxygen, simultaneously forming a superoxide ion. The anionic carboxylate group improves the stability of the bound flavonoxy radical by providing substantial electron density to the electron-deficient fla˙ through the Co(II) centre, allowing the reactive fla˙ species to accumulate at an optimal concentration for effective catalysis. EPR spectroscopy successfully detected the cobalt-bound fla˙ species formed through the dioxygenation of 1fla. NBT2+ and EPR spin-trapping experiments confirmed superoxide formation during the dioxygenation process. So, the present work describes CoII-2,4-QD model studies and clarifies the function of carboxylate in quercetinase-like reactivity.


 Please wait while we load your content...
Please wait while we load your content...