Enhanced hydrogen storage kinetics of MgH2 by the synergistic effect of Mn3O4/ZrO2 nanoparticles
Abstract
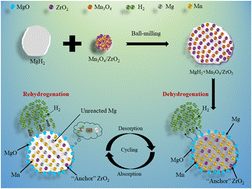
As an ideal material for solid-state hydrogen storage, magnesium hydride (MgH2) has attracted enormous attention due to its cost-effectiveness, abundant resources, and outstanding reversibility. However, the high thermodynamics and poor kinetics of MgH2 still hinder its practical application. In this work, a simple stirring–hydrothermal method was used to successfully prepare bimetallic Mn3O4/ZrO2 nanoparticles, which were subsequently doped into MgH2 by mechanical ball milling to improve its hydrogen sorption performance. The MgH2 + 10 wt% Mn3O4/ZrO2 composite began discharging hydrogen at 219 °C, which was 111 °C lower compared to the as-synthesized MgH2. At 250 °C, the MgH2 + 10 wt% Mn3O4/ZrO2 composite released 6.4 wt% hydrogen within 10 min, whereas the as-synthesized MgH2 reluctantly released 1.4 wt% hydrogen even at 335 °C. Moreover, the dehydrogenated MgH2 + 10 wt% Mn3O4/ZrO2 sample started to charge hydrogen at room temperature. 6.0 wt% hydrogen was absorbed when heated to 250 °C under 3 MPa H2 pressure, and 4.1 wt% hydrogen was taken up within 30 min at 100 °C at the same hydrogen pressure. In addition, compared with the as-synthesized MgH2, the de/rehydrogenation activation energy values of the MgH2 + 10 wt% Mn3O4/ZrO2 composite were decreased to 64.52 ± 13.14 kJ mol−1 and 16.79 ± 4.57 kJ mol−1, respectively, which incredibly contributed to the enhanced hydrogen de/absorption properties of MgH2.


 Please wait while we load your content...
Please wait while we load your content...