Accurate single crystal and gas-phase molecular structures of acenaphthene: a starting point in the search for the longest C–C bond†
Abstract
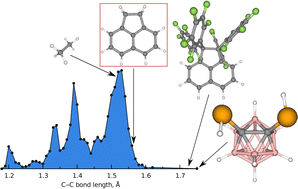
The molecular structure of acenaphthene has been determined experimentally in the gas phase using gas electron diffraction intensities and literature-available rotational constants. Supplementary high-level quantum-chemical calculations were utilized in refinements of the semi-empirical equilibrium structure. In this work we investigate on how different schemes of GED data averaging and weighting can be used for obtaining the most accurate and precise structural parameters. Single-crystal X-ray diffraction experiments at different temperatures have been performed and the solid-state structure of acenaphthene has been determined. Both gas and solid-state acenaphthene molecules are planar and possess a non-twisted ethylene bridge. The aliphatic C–C bond in the ethylene fragment is elongated to 1.560(4) Å in the gas phase and 1.5640(4) Å in the solid phase. Based on the experimental data several theoretical approximations have been calibrated and predictions for other molecules were made, taking into account dispersion and electrostatic interactions. Particular derivatives of acenaphthene may potentially have significantly elongated C–C bonds up to 1.725 Å. However, among the experimental gas-phase structures available to date probably the longest C–C bond (re,(av) = 1.750(28) Å at w = 0.93) was determined in a carbaborane derivative 1,2-(SeH)2-closo-1,2-C2B10H10.


 Please wait while we load your content...
Please wait while we load your content...