Theoretically seeking charge transport materials with inherent mobility higher than 2,6-diphenyl anthracene: three isomers of 2,6-dipyridyl anthracene†
Abstract
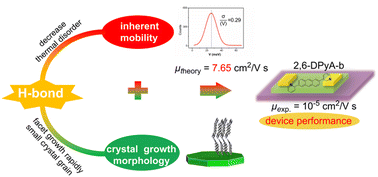
2,6-Diphenyl anthracene (2,6-DPA) is a well-known anthracene derivative with high hole mobility (34 cm2 V−1 s−1) among p-type organic semiconductors (OSCs). In contrast, three 2,6-dipyridyl anthracene (2,6-DPyA) molecules (ortho-, meta-, and para-pyridyl), which are isoelectronic to 2,6-DPA showed relatively low mobility in experiments. To explore the origin of different charge transport properties and gain new inspiration on the design of novel organic semiconductor materials, the intrinsic hole transport property of 2,6-DPA and three isomeric 2,6-DPyAs were theoretically investigated and compared by quantum-chemical methodology and molecular dynamics simulation. The calculated results indicate that the intrinsic mobility of 2,6-DPyA-b (meta-) is superior to that of 2,6-DPA (12.73 vs. 3.54 cm2 V−1 s−1). Furthermore, the possibility that 2,6-DPyA-b may be strongly affected by thermal fluctuations is excluded because of the strong intermolecular C–H⋯N interactions (H-bonds). In addition, the crystal growth morphology prediction is considered in depth by the attachment energy (AE) model. The prediction results demonstrate that the strong intermolecular H-bonds in 2,6-DPyA do not facilitate the formation of a large and regular crystal face but rather the production of many grains and grain boundaries, which is not conducive to the charge carrier transport. This study reflects the paradox of the H-bond in OSCs and highlights the indispensability of the mesoscopic crystal growth morphology prediction in identifying high performance OSC materials and the establishment of the relationship between microcosmic organic molecules and macroscopic device performance.


 Please wait while we load your content...
Please wait while we load your content...