Binding kinetics study of SARS-CoV-2 main protease and potential inhibitors via molecular dynamics simulations†
Abstract
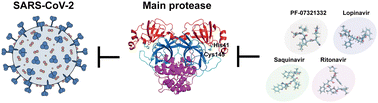
The pandemic COVID-19 was induced by the novel coronavirus SARS-CoV-2. The virus main protease (Mpro) cleaves the coronavirus polyprotein translated from the viral RNA in the host cells. Because of its crucial role in virus replication, Mpro is a potential drug target for COVID-19 treatment. Herein, we study the interactions between Mpro and three HIV-1 protease (HIV-1 PR) inhibitors, Lopinavir (LPV), Saquinavir (SQV), Ritonavir (RIT), and an inhibitor PF-07321332, by conventional and replica exchange molecular dynamics (MD) simulations. The association/dissociation rates and the affinities of the inhibitors were estimated. The three HIV-1 PR inhibitors exhibit low affinities, while PF-07321332 has the highest affinity among these four simulated inhibitors. Based on cluster analysis, the HIV-1 PR inhibitors bind to Mpro at multiple sites, while PF-07321332 specifically binds to the catalytically activated site of Mpro. The stable and specific binding is because PF-07321332 forms multiple H-bonds to His163 and Glu166 simultaneously. The simulations suggested PF-07321332 could serve as an effective inhibitor with high affinity and shed light on the strategy of drug design and drug repositioning.


 Please wait while we load your content...
Please wait while we load your content...