First and second sphere interactions accelerate non-native N-alkylation catalysis by the thermostable, methanol-tolerant B12-dependent enzyme MtaC†
Abstract
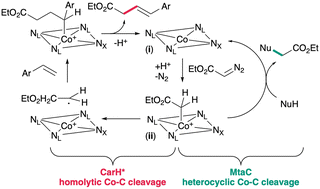
The corrinoid protein MtaC, which is natively involved in methyl transferase catalysis, catalyzes N-alkylation of aniline using ethyl diazoacetate. Our results show how the native preference of B12 scaffolds for radical versus polar chemistry translates to non-native catalysis, which could guide selection of B12-dependent proteins for biocatalysis. MtaC also has high thermal stability and organic solvent tolerance, remaining folded even in pure methanol.
- This article is part of the themed collection: Biocatalysis: A cross-journal collection


 Please wait while we load your content...
Please wait while we load your content...