Integrated recycling of valuable elements from spent LiFePO4 batteries: a green closed-loop process†
Abstract
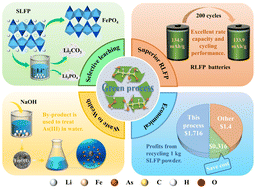
The harmless disposal and resourceful recovery of spent lithium-ion batteries is an inevitable choice to protect the environment, conserve resources and promote the development of the circular economy. A systematic, green, and sustainable recycling process for waste LiFePO4 batteries is proposed based on malic acid. The method employs naturally degradable organic acids instead of traditional inorganic acid leaching, reducing the negative impact on the environment. Under optimized conditions, 99.12% Li is extracted, while less than 1% Fe is leached. This fraction of iron ions is cleverly employed as a catalyst to promote the leaching efficiency of lithium. Furthermore, the iron by-products from the purification process are used for As(III) adsorption and show surprising arsenic removal properties. A minor amount of P in the leachate is recovered as Li3PO4, and most Li is collected as Li2CO3 with 99.63% purity. Ultimately, the LiFePO4 cathode material is regenerated from the obtained Li2CO3 product and FePO4 residue. Compared with the traditional method, this process merits efficient lithium–iron separation, environmental friendliness, and economic efficiency.


 Please wait while we load your content...
Please wait while we load your content...