One-dimensional water channels in lanthanum sulfate: a first-principles study†
Abstract
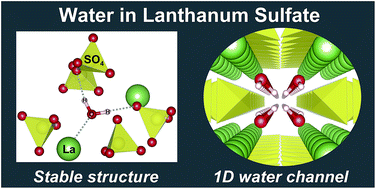
The stable structure and diffusion mechanism of water in lanthanum sulfate La2(SO4)3 have been theoretically analyzed in a first-principles manner based on the density functional theory (DFT). The first-principles molecular dynamics (FPMD) simulation indicates that water in the sulfate crystal is dissolved and migrates with maintaining the shape of the water molecule. In this simulation, three elementary processes for water diffusion were observed (paths 1–3), whose potential barriers were calculated to be 0.27, 0.81, and 1.50 eV, respectively, by potential energy surface (PES) mapping. Path 1 with the lowest potential barrier is only oscillatory transfer between adjacent sites, while path 2 with the second-lowest potential barrier forms one-dimensional (1D) water channels in the [010] direction. The water diffusivity along the channels is estimated to be ∼10−10 cm2 s−1 at 523 K, leading to a diffusion length in the micrometer range within a few minutes. This suggests that the reported fast hydration/dehydration reaction of La2(SO4)3 should be attributed not only to the 1D water channels in the bulk but also to the small crystal grain size and the high grain boundary diffusivity.


 Please wait while we load your content...
Please wait while we load your content...