A striking catalytic effect of facile synthesized ZrMn2 nanoparticles on the de/rehydrogenation properties of MgH2†
Abstract
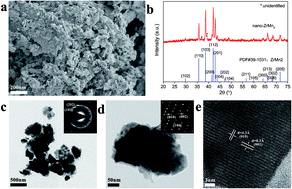
Zr-based Laves phase alloys, especially ZrMn2, have been widely studied because of their good hydrogen storage properties. In this work, ZrMn2 nanoparticles were successfully prepared by a facile wet-chemical method and then introduced to MgH2via ball milling to fabricate MgH2–ZrMn2 composites. Remarkable improvements to the de/rehydrogenation properties were achieved with the addition of ZrMn2 nanoparticles. The MgH2 + 10 wt% nano-ZrMn2 composite started to release hydrogen at 181.9 °C, which was about 160 °C lower compared with that of additive-free MgH2. At 300 °C, the MgH2+ 10 wt% nano-ZrMn2 composite desorbed 6.7 wt% hydrogen in 5 min. More importantly, the dehydrogenated MgH2 + 10 wt% nano-ZrMn2 sample absorbed hydrogen even at room temperature under 3 MPa hydrogen pressure, and approximately 5.3 wt% hydrogen was taken up within 10 min at 100 °C. Moreover, compared with additive-free MgH2, the dehydrogenation and rehydrogenation activation energies of the MgH2 + 10 wt% nano-ZrMn2 composites were significantly reduced to 82.2 ± 2.7 kJ mol−1 and 22.1 ± 2.7 kJ mol −1, respectively. TEM analysis demonstrated the uniform distribution of ZrMn2 nanoparticles in the MgH2 matrix. Further, density functional theory calculations revealed that the presence of ZrMn2 facilitated the breaking of the Mg–H bond, which provided a good explanation for the reduced de/rehydrogenation temperatures of the ZrMn2 modified MgH2.


 Please wait while we load your content...
Please wait while we load your content...