High surface area MnO2 nanomaterials synthesized by selective cation dissolution for efficient water oxidation†
Abstract
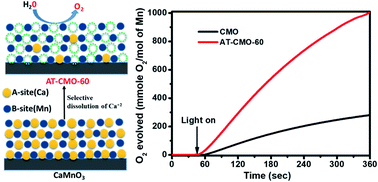
Artificial photosynthesis is a promising method that directly transforms solar energy into chemical energy. To achieve artificial photosynthesis, efficient water oxidation catalysts (WOCs) are essential. In nature, the manganese-oxo-calcium cluster (Mn4CaO5) in the oxygen-evolving center (OEC) of photosystem II catalyzes water oxidation. Inspired by this process, abundant and inexpensive manganese oxides have been recognized for their high potential as effective and reliable materials for water oxidation reaction. However, in contrast to RuO2 and IrO2, manganese oxide catalysts still exhibit less water oxidation efficiency. Herein, we report a simple method for synthesizing high surface area, porous MnO2 with different crystal structures from the CaMnO3 precursor by treating with dilute HNO3 solution followed by heating at different temperatures. These porous MnO2 nanomaterials have a surface area in the range of 106–272 m2 g−1. These nanomaterials exhibit remarkable photochemical water oxidation activity with a maximum turnover frequency (TOF) of 3.29 × 10−3 s−1. Our findings suggest a simple way to design and synthesize low-cost, high surface area, porous MnO2 nanostructures for efficient water oxidation.


 Please wait while we load your content...
Please wait while we load your content...