Highly specific fluorescent probes toward acetic acid via structural transformation of zinc complexes†
Abstract
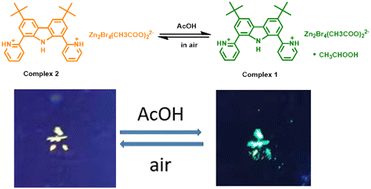
A highly sensitive and specific acetic acid probe is desirable to distinguish acetic acid from other fatty acids. Herein, we designed and synthesized two zinc complexes, H2tBuCarPyZn2Br4(AcO)2·AcOH (1) and H2tBuCarPyZn2Br4(AcO)2 (2). Both complexes exhibit fluorescence with emission peaks at 500 nm for complex 1 and 550 nm for complex 2, respectively. The DFT calculation shows that the transition from Br to the H2tBuCarPy ligand is mainly responsible for the yellow emission of complex 2, while for complex 1 the valence band consists of Br and acetic acid solvent, and the participation of acetic acid in complex 1 could result in the blue-shift of emission compared with that of complex 2. Interestingly, an obvious blue-shift of emission was observed when complex 2 was exposed to an acetic acid atmosphere. Besides, the as-exposed complex 2 could recover to the initial state once the acetic acid atmosphere was removed. PXRD results demonstrate that the phase change from complex 2 to complex 1 after absorbing the acetic acid vapor accounts for the vapor-chromism. Then complex 2 was developed as an acetic acid probe, exhibiting high sensitivity and selectivity and excellent fatigue resistance.
- This article is part of the themed collection: Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...