A phenothiazine-fused electroactive bilayer helicene: design, synthesis, ACQ-to-AIE transformation and photophysical properties†
Abstract
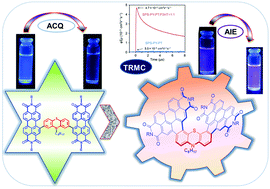
π-Extended helicenes and their derivatives represent green polycyclic aromatic hydrocarbons. Here, we report the design and synthesis of a novel phenothiazine and perylene diimide (PDI)-fused [7]helicene, represented as SPS-PY-PT, to study the aggregation-induced emission (AIE) phenomenon along with its photophysical and electroactive properties. The conventional strategy for AIE uses a twisted molecule or core-connected twisted molecules. However, the present strategy has shown perylene itself as a twisted structure in SPS-PY-PT, which shows effective AIE properties. In contrast, uncyclized PY-PT-Un shows aggregation-caused quenching (ACQ) rather than the AIE phenomenon. Further, the photoconductive properties of SPS-PY-PT were examined by time-resolved microwave conductivity (TRMC). The transient photoconductivity shows a great increase on mixing with a poly-3-hexylthiophene (P3HT) polymer donor, indicating an efficient electron transfer from P3HT to SPS-PY-PT. Electrochemical analyses are conducted and correlated with theoretical calculations. The photophysical and electrochemical data suggest that SPS-PY-PT can be used as an electron acceptor for organic photovoltaics and additionally gives a direction towards AIE-based sensors.
- This article is part of the themed collection: Organic Electronics – Ecofriendly and/or sustainable materials, processes, devices, and applications


 Please wait while we load your content...
Please wait while we load your content...