Phase transition with in situ exsolution nanoparticles in the reduced Pr0.5Ba0.5Fe0.8Ni0.2O3−δ electrode for symmetric solid oxide cells†
Abstract
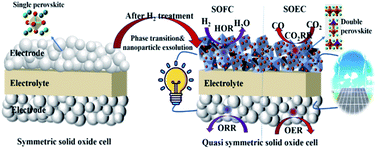
Symmetric solid oxide cells (SSOCs) have attracted enormous attention in research and development because of their simple cell configuration and low fabrication costs. However, their development is limited by their electrocatalytic activity and stability of the electrode materials used. Herein, we report a novel perovskite oxide electrode Pr0.5Ba0.5Fe0.8Ni0.2O3−δ (PBFN) as a highly effective SSOC electrode material. The results demonstrate that PBFN has outstanding electrocatalytic potential for the oxygen reduction reaction, oxygen evolution reaction, carbon dioxide reduction reaction, and hydrogen oxidation reaction. After H2 treatment, its structure changes from single to double perovskite and is accompanied by Fe–Ni alloy nanoparticle exsolution. Compared with PBFN, the SSOCs with reduced PBFN qualities show improved electrochemical performance. A reduced PBFN-based device has a higher power density (0.201 W cm−2vs. 0.151 W cm−2 for H2 as a fuel at 750 °C) and an electrolysis current density (0.524 A cm−2vs. 0.353 A cm−2 for the electrolysis of pure CO2 at 750 °C @ 2 V) in comparison to the PBFN approach as is the case with other symmetric cell results. Thus, the reduced PBFN-based device shows favorable stability for both power density and electrolysis modes. These results suggest that phase transition and nanoparticle exsolution is a promising strategy for high performance SSOCs.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...