Recycling alloy scrap and CO2 by paired molten salt electrolysis†
Abstract
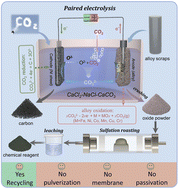
Reclaiming valuable materials from retired alloys and CO2 in an eco-friendly and efficient manner is critically important to meet the urgent need for strategical metals and curb climate change. Herein, we report a molten salt electrolyzer with an alloy scrap anode along with a CO2 reduction cathode for co-recycling alloy scrap and CO2. In molten CaCl2–NaCl–CaCO3 at 700 °C, the alloy scrap is converted to porous oxide scale at the anode and CO2 is reduced to carbon at the cathode. The Cl− in the molten salt prevents the passivation and allows the continuous oxidation of the alloy scrap anode, and CO32− was electrochemically reduced to provide O2− while generating carbon. The average particle size of the anode oxides is about 8 μm and the cathode carbon is amorphous structure. Furthermore, the obtained oxides can be converted to water-soluble sulfates by sulfation roasting with recovery rates of over 92% for both Co and Ni. More importantly, the paired electrolysis method improves energy efficiency by repurposing both waste alloy scrap and CO2. This paper provides a promising and green strategy to recover valuable metals from various alloy scrap and C/CO from carbon dioxide to close both metal and carbon cycles.
- This article is part of the themed collection: Sustainable Energy & Fuels Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...