Thermodynamic analysis of the electrochemical synthesis of ammonia in solid-state proton-conducting electrochemical reactors considering interfacial potential steps
Abstract
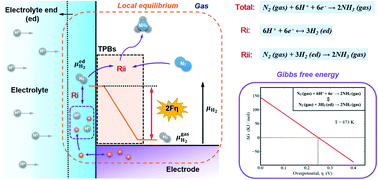
Ammonia (NH3) as a carbon-free hydrogen carrier shows great potential as fuel and its production under mild conditions is desired. NH3 synthesis at atmospheric pressure can be realized in solid-state proton-conducting electrochemical reactors (PCERs), in which the electrochemically pumped hydrogen driven by potential steps at the electrolyte–electrode interface plays an important role. However, until now, only qualitative statements are available and the quantitative analysis of the thermodynamic activity of hydrogen at the interface of the electrode/electrolyte is absent. Therefore, in this study, considering the interfacial effect of potential steps at the electrode–electrolyte interfaces and the chemical potentials (thermodynamic activity) of gas species within the electrolyte layer, an electrochemical model of PCER is developed to quantitatively elucidate the interfacial effect of potential steps. The mathematical relationship between the thermodynamic feasibility of electrochemical synthesis and interfacial potential steps is established. The modeling results indicate that the energy of interfacial potential steps can transform into the electrochemical potential of proton at the end of the electrolyte layer, leading to significant growth of the electrochemical potential of proton (equivalent PH2 up to 103 atm). Such a high PH2 makes the ΔG of ammonia synthesis become negative, demonstrating the thermodynamic feasibility of the electrochemical synthesis of ammonia at atmospheric pressure. This work reveals the role of interfacial potential steps in hydrogen activation in PCERs, which can serve as the basic thermodynamic principles for ammonia synthesis and hydrogenation reactions in PCERs.
- This article is part of the themed collection: Sustainable Energy & Fuels Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...