Probing the methanol-assisted autocatalytic formation of methanol over Cu/ZnO/Al2O3 by high-pressure methanol and methyl formate pulses†
Abstract
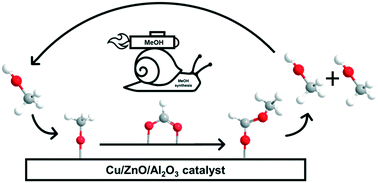
Using high-pressure methanol and methyl formate pulses as a surface-sensitive operando method for high-pressure methanol synthesis over Cu/ZnO/Al2O3, the recently found autocatalytic pathway was confirmed. The autocatalytic effect is assumed to result from the faster hydrogenation of the formed methyl formate ester at high methoxy coverages compared with the rate-determining hydrogenation of formate to dioxomethylene. When pulsing increasing amounts of methanol at 60 bar and 210 °C under kinetically controlled conditions in 13.5 vol% CO, 3.5 vol% CO2, and 73.5 vol% H2, higher amounts of methanol were observed in response. The surplus of formed methanol was found to increase exponentially as a function of the dosed amount of methanol and the applied residence time. To further investigate the methanol-assisted autocatalytic pathway, methyl formate as the predicted intermediate was pulsed, which was rapidly converted into methanol. Instead of the expected 2 : 1 stoichiometry of methanol : methyl formate, only one methanol molecule was produced per dosed methyl formate molecule. It is concluded that methyl formate is split into methoxy and formate species by dissociative adsorption, but only methoxy species are rapidly further hydrogenated to desorbing methanol, whereas formate hydrogenation to methanol is too slow on the time scale of the pulse experiments.


 Please wait while we load your content...
Please wait while we load your content...