A visible light-mediated three-component strategy based on the ring-opening of cyclic ethers with aryldiazoacetates and nucleophiles†
Abstract
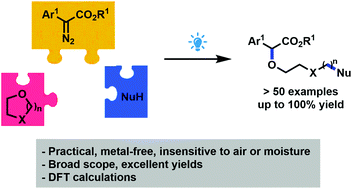
A blue light-promoted reaction between aryldiazoacetates and different nucleophiles has been developed in the presence of THF (and other cyclic ethers) as the solvent, allowing the incorporation of these three elements into a single product. Formally, this transformation represents an O–H insertion strategy of more complex alcohols into aryldiazoesters, without the need of pre-assembling them. The method is mild and efficient and proceeds in the absence of metals, generally at room temperature.


 Please wait while we load your content...
Please wait while we load your content...