Nonstoichiometric hydroarylation polyaddition for synthesis of pyrrole-based poly(arylenevinylene)s†
Abstract
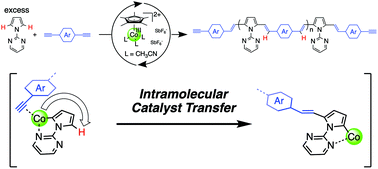
Nonstoichiometric polyaddition via the Co-catalyzed hydroarylation of diyne monomers with excess 1-(2-pyrimidinyl)pyrrole was demonstrated. The 2-pyrimidinyl substituent as a directing group promoted intramolecular catalyst transfer, producing the corresponding poly(arylenevinylene)s with alkyne terminals at both ends and a high molecular weight.


 Please wait while we load your content...
Please wait while we load your content...