Cationic ring-opening polymerization of a five membered cyclic dithiocarbonate having a tertiary amine moiety†
Abstract
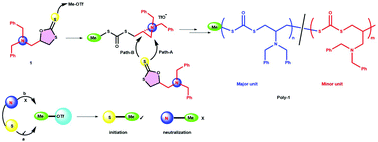
In this work, a facile route for the cationic ring-opening polymerization (CROP) of a cyclic dithiocarbonate derived from N,N-dibenzyl glycidylamine (DBGA) and carbon disulfide has been developed. The cyclic dithiocarbonate monomer, 5-((dibenzylamino)methyl)-1,3-oxathiolane-2-thione (1), can be obtained economically and easily in three steps from commercially available N,N-dibenzylamine. The cationic polymerization of 5-((dibenzylamino)methyl)-1,3-oxathiolane-2-thione (1) was achieved using methyl triflate or ethyl triflate as an initiator. The polymerization proceeded smoothly in chlorobenzene with high monomer conversion (>90%). The polymers exhibited a high molar mass with narrow dispersity. The structure of the polymers was confirmed by NMR and IR analyses. Investigation of the mechanism of this polymerization by NMR studies on the stoichiometric reaction of 1 with MeOTf indicates the higher reactivity of sulfur than that of nitrogen for the initiator (methyl cation, S > N). A strong possibility of formation of a transient aziridinium ion by neighboring group participation of the tertiary amine moiety is also seen. Finally evidence from DFT calculation also clarifies the presence of more negative charge on the sulfur atom of the thiocarbonyl group compared to nitrogen.
- This article is part of the themed collection: Synthetic Methodologies for Complex Macromolecular Structures


 Please wait while we load your content...
Please wait while we load your content...