Synthesis of 6′-galactosyllactose, a deviant human milk oligosaccharide, with the aid of Candida antarctica lipase-B†
Abstract
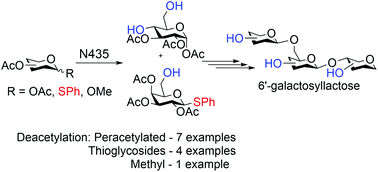
Research on human milk oligosaccharides (HMOs) has increased over the past decade showing great interest in their beneficial effects. Here we describe a method for the selective deacetylation using immobilised Candida antarctica lipase-B, Novozyme N435 (N435), of pyranose saccharides in organic media with the aim of simplifying and improving the pathways for the synthesis of HMOs. By first studying in depth the deacetylation reaction of peracetylated D-glucose two reaction conditions were found, which were used on different HMO building blocks, peracetylated saccharides and thioglycosides. D-Glucose based saccharides showed selectivity towards the fourth and the sixth position deacetylation. While α-anomer of peracetylated D-galactose remained unreactive and β-anomer favoured the first position deacetylation. Peracetylated L-fucose, on the other hand, had no selectivity as the main product was fully unprotected L-fucose. Taking the peracetylated D-glucose deacetylation reaction product and selectively protecting the primary hydroxyl group in the sixth position left only the fourth position open for the glycosylation. Meanwhile, the deacetylation product of D-galactose thioglycoside, with the sixth position deacetylated, had both acceptor and donor capabilities. Using the two aforementioned products derived from the N435 deacetylation reactions a deviant HMO, 6′-galactosyllactose (6′-GL) was synthesised.


 Please wait while we load your content...
Please wait while we load your content...