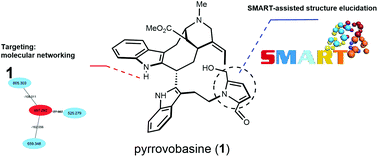
A new vobasine–tryptamine-based monoterpene indole alkaloid pseudodimer was isolated from the stem bark of Voacanga africana. As a minor constituent occurring in a thoroughly investigated plant, this molecule was targeted based on a molecular networking strategy and a rational MS2-guided phytochemical investigation led to its isolation. Its structure was formally established based on HRMS, 1D/2D NMR data, and the application of the tool Small Molecule Accurate Recognition Technology (SMART 2.0). Its absolute configuration was assigned by the exciton chirality method and TD-DFT ECD calculations. Besides featuring an unprecedented intermonomeric linkage in the small group of vobasine/tryptamine hybrids, pyrrovobasine also represents the first pyrraline-containing representative in the whole monoterpene indole alkaloids group. Biosynthetic hypotheses possibly underpinning these structural oddities are proposed here.