Reactivity switch-over of 4-hydroxydithiocoumarins under various conditions and their application in organic synthesis†
Abstract
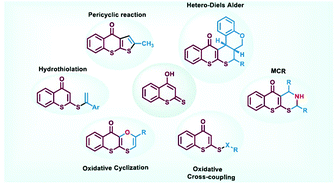
4-Hydroxydithiocoumarin is a valuable organic precursor to architect important heterocycles. The three different reactive nucleophilic sites in 4-hydroxydithiocoumarins display intriguing regioselectivity in their reaction towards various electrophiles. Previously, 4-hydroxydithiocoumarins have been used in the synthesis of heterocycles using Claisen and thio-Claisen reactions. Hetero-Diels–Alder reactions involving 4-hydroxydithiocoumarins have proven to be very convenient in assembling complex molecular organic frameworks from readily available feedstocks. Development of multicomponent reactions employing 4-hydroxydithiocoumarins has given rise to several important reaction protocols to access polycyclic compounds. Recently, this moiety has been used in forging some unusual S–S, S–N and S–O bonds under oxidative conditions which further explores its hidden reactivity in organic synthesis. Besides that, hydrothiolation of various alkynes and alkenes using 4-hydroxydithiocoumarins has led to the synthesis of some potential lead molecules. This mini-review provides an account of the reactivity pattern of 4-hydroxydithiocoumarins and their strategic applications in various reactions for the synthesis of several heterocycles and other important organic syntheses.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...