Rh(i)-catalyzed site-selective hydroacylation of alkenyl-bearing allylic alcohols with non-chelating aldehydes controlled by in situ generated carbonyl group†
Abstract
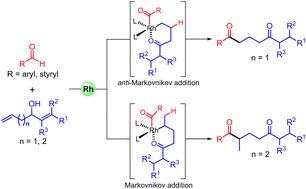
A rhodium(I)-catalyzed regioselective hydroacylation of alkenyl-bearing allylic alcohols with simple aldehydes for the preparation of diverse 1,5-diketones is described. Mechanistic investigation suggests that this transformation might proceed through redox isomerization of the allylic alcohol followed by hydroacylation of the resulting enone with aldehyde involving a stable six-membered rhodacycle intermediate. This protocol highlights the role of in situ generated carbonyl group in controlling the site-selectivity of the intramolecular alkene moiety.


 Please wait while we load your content...
Please wait while we load your content...