Hexaphenylbenzene-based fluorescent probes for the detection of fluoride ions†
Abstract
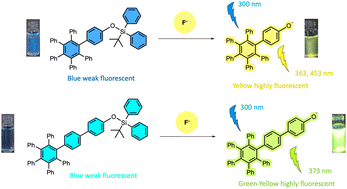
Novel hexaphenylbenzene derivatives (HPB-1 and HPB-2) were synthesized and their structural and optical properties were characterized using 1H NMR, 13C NMR, HRMS, absorption and fluorescence spectroscopies. The effect of various anions (AcO−, NO3−, HSO4−, ClO−, ClO4−, SCN−, CN−, F−, Cl−, Br−, I− and H2PO4−) on the optical properties of these new compounds was investigated in THF. Among the different anions, significant changes in the fluorescence spectra of HPB-1 and HPB-2 were observed in the presence of only F− ions. The interaction ratios of HPB-1 and HPB-2 with F− ions were determined to be 1 : 1 and the binding constants (KA) were found to be 0.0417 M−1 and 0.982 M−1 for HPB-1 and HPB-2, respectively. The LOD values were calculated as 2.79 μM and 1.04 μM for HPB-1 and HPB-2, respectively. The mechanism of F−-ion sensing was investigated via1H NMR titration. The obtained results show that the newly synthesized HPB-1 and HPB-2 compounds can be a turn-on fluorometric sensor with high selectivity for F− ions.


 Please wait while we load your content...
Please wait while we load your content...