Meso bromination and derivatization of synthetic bacteriochlorins†
Abstract
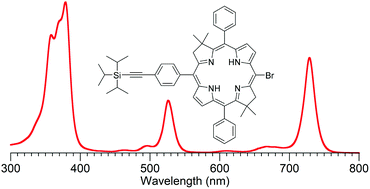
The ability to prepare and tailor synthetic analogues of native bacteriochlorophylls enables diverse applications. A de novo route entails dimerization of a dihydrodipyrrin-acetal to afford the corresponding 5-methoxy and/or 5-unsubstituted bacteriochlorin, wherein each pyrroline ring contains a gem-dimethyl group to ensure stability toward adventitious dehydrogenation. The presence of a 5-methoxy group facilitates bromination at the distal meso-(15-)position. While bromination of 5-unsubstituted bacteriochlorins typically affords a mixture of brominated products, here the presence of two substitution patterns (2,12-dicarboethoxy, 2,12-diacetyl) has been found to facilitate selective meso-bromination in the absence of the methoxy substituent. The introduction of a single meso-bromine atom in a bacteriochlorin opens opportunities for Pd-mediated derivatization, which include (1) preparation of four ethynylphenyl building blocks (and two benchmark bacteriochlorins) with long-wavelength absorption bands tuned across 725–757 nm, for use in preparation of multichromophore arrays; (2) installation of a bioconjugatable group to free base bacteriochlorins or a copper bacteriochlorin, the latter for possible use in photoacoustic imaging; and (3) installation of an S-acetylthio group for surface attachment. Altogether, 25 new bacteriochlorins are described including 5 meso-bromobacteriochlorin intermediates and 12 target bacteriochlorins.


 Please wait while we load your content...
Please wait while we load your content...
